Journal of
eISSN: 2378-3184


Research Article Volume 8 Issue 4
Far Eastern State Technical Fisheries University, Russia
Correspondence: Nikolay N Kovalev, Far Eastern State Technical Fisheries University, Vladivostok, 690087, Russia
Received: August 13, 2019 | Published: August 26, 2019
Citation: Kovalev NN, Pozdnyakova YM, Leskova SE, et al. Effect of nucleotide-containing feed on growth and biochemical parameters of juvenile Japanese sea cucumber, Apostichopus japonicus (selenka, 1867) (Echinodermata: Holothuroidea). J Aquac Mar Biol. 2019;8(4):139-142. DOI: 10.15406/jamb.2019.08.00252
The effect of multicomponent feed formulae on body weight and survival rate of juvenile Japanese sea cucumber was studied at a hatchery farm. It has been found that the growth rate of juveniles exposed to a constant water temperature and salinity is high. DNA from salmon milt, included in the feed formula at a concentration of 1mg/kg, exhibits a stimulating effect on juveniles’ growth parameters. A higher DNA content of feed (5mg/kg) reduces its feed conversion ratio. Salmon milk DNA was manufactured by salt extraction and ethyl alcohol precipitation. The product is a fragment of nucleoproteins with a mass of about 300kDa.
The seasonal water freshening causes the growth rate and the rate of feed consumption to decrease. The introduction of DNA in feed contributes to the accumulation of larger amounts of protein and hexosamines in tissues of juvenile sea cucumbers as compared to the control.
Keywords: Japanese sea cucumber, aquaculture, feed, DNA, oligonucleotides, growth rate
The growing world-wide demand for Japanese sea cucumber has caused an increase in the harvesting pressure on its natural populations and, as a consequence, a significant overfishing. Due to the depletion of natural resources, as well as the high commercial value, there was an urgent necessity to implement aquaculture programs for holothurians. The successful incubation of juvenile Apostichopus japonicus (Selenka, 1867) in the 1980s facilitated the development of aquaculture of the species in Japan and China. The increase in species stocks due to the release of juveniles was a reasonable solution aimed at recovery of natural populations.1
The major issue of sea cucumber farming is the lack of feed and scientifically substantiated dietary patterns. At the same time, calculation of energy budget provides a basis for evaluating different approaches to the use of feed nutrients. To date, very few models are known to calculate the energy of feed proposed for echinoderms.2
Differences in feed formulae are usually associated with the species of algae used, the quality of protein, and biologically active components introduced. Micronutrients play the role of growth stimulators, or components that provide higher survival rate of juveniles under conditions of aquarium-based rearing and aquaculture. Nevertheless, information on nutritional demands and preferred rations of juvenile Japanese sea cucumber is extremely limited, which hinders the development of methods for hatchery cultivation.
The goal of the study was to assess the effect of feed formulae containing DNA extracted from salmon milt on the growth and chemical composition of juvenile sea cucumber.
The object of the study was hatchery-reared juvenile Japanese sea cucumbers obtained at the experimental sea cucumber cultivation facility in Severnaya Cove (Slavyanka Bay, Primorsky Krai). The juvenile sea cucumbers were kept in tanks with running water at a stocking density of 0,3 individuals per l liter. The animals were fed twice a day at a rate of 100mg of feed per individual.
The composition of the feed formula included dried kelp, fish flour, soybean meal, crushed bivalve shells, and freeze-dried sea cucumber internals at a ratio of 4:2:1:3:0.05. Flour is obtained by direct drying of minced meat of Alaska Pollock, contains (in %): protein 85-86, fat- 1,5-2,0 and ash content of 3.5-4.5. The feed formulae were supplemented with DNA as a biologically active component in the form of nucleoprotein complex from salmon milt at a concentration of 1g (feed no. 1) and 5g (feed no. 2) per 1kg of feed weight. Dry animal feed is produced by sintering method. Sintering was performed at 1000C within 30 minutes. When sintering the powder particles of the prescription mixture stick together under the influence of temperature, the air goes out, the mixture shrinks and compacts.
The basic feed formula was the control. The biologically active additive was obtained by salt extraction and ethyl alcohol precipitation. The product is a fragment of nucleoproteins with a mass of about 300kDa.3
The experimental studies were conducted for 101 days, from June to September. Water temperature and salinity were measured three times a day using an electronic thermometer and salinometer. Feed efficiency was assessed by variations in the body weight of the juvenile sea cucumbers.
Experimental data were processed based on the following parameters: survival rate, specific growth rate, feed consumption rate, and efficiency of food conversion. The parameters were calculated as follows4
where N1 is the number of animals at the beginning of experiment, N2 is number of animals at the end of experiment, W1 and W2 are the initial and final values of sea cucumber weight in each of the experimental tanks, Т is the experimental period, and I is the dry weight of feed supplied to the tank.
The chemical composition of the muscular sac of sea cucumbers was identified based on the quantitative content of water-soluble proteins and hexosamines in the sea cucumber muscular sac. Sample preparation for the determination of water-soluble protein was carried out by extraction of homogenized tissue in distilled water in a ratio of 1 mg/ml for 1 h at a temperature of 25°C. The extract was filtered through a paper filter. Protein content was determined in the filtrate by the method of Lowry et al.5 The content of hexosamines was determined spectrophotometrically. The method is based on the interaction of hexosamines with an alkaline solution of acetylacetone at a pH of 9.6-9.7 when heated, followed by treatment with Erlich reagent. The optical density of the red-colored solution is measured at 530nm.6
The efficiency of feed with the DNA content of 1 mg (feed no. 1) and 5mg (feed no. 2) per 1kg of feed weight was assessed in comparison with a feed without DNA (control). Each experimental group of juvenile sea cucumbers was kept separately in 100-lilter tanks. To estimate variations in the body weight of juveniles, control weighing was carried out monthly. The animals were taken out from the water, dried on filter paper, and weighed on an electronic scale to an accuracy of 0.01g. After the weight measurements, the juveniles were put back in the same tanks where they were kept for further experimental cultivation. Survival rate was estimated by the number of juveniles that survived in each experiment. The number of animals by the beginning of the experiment was 30 in each group.
The quantitative data on body weight variations and number of live juvenile sea cucumbers are presented in Table 1.
|
Date, parameter |
Feed no. 1 |
Feed no. 2 |
Control |
|
June 9 |
n=30 |
n=30 |
n=30 |
| Weight, g | 0.23±0.1 |
0.38±0.25 |
0.25±0.15 |
|
July 9 |
n=23 |
n=25 |
n=26 |
|
Weight, g |
0.94±0.18 |
1.45±0.10 |
1.32±0.20 |
|
August 12 |
n=23 |
n=22 |
n=25 |
|
Weight, g |
3.5±0.38 |
2.83±0.24 |
2.72±0.17 |
|
September 20 |
n=21 |
n=21 |
n=24 |
|
Weight, g |
2.22±0.48 |
1.36±0.28 |
2.04±0.17 |
Table 1 The effect of feed formula on the body weight of juvenile sea cucumbers
The data in the table show that the use of experimental feed formulae contributed to the body weight increase in the experimental animals. However, the increment in animals’ weight during the 101 days of the experiment had a non-linear pattern. Thus, within the first month of the experiment, the weight of juvenile sea cucumbers in the experimental groups 1 and 2 increased 4.1- and 3.8-fold, respectively; in the control group, 5.3-fold. From July to August, the growth rate of juveniles reduced and amounted to 1.38g for the group 2 and 1.4g for the control group, respectively. At the same time, the body weight of the experimental group 2 increased by 2.56g. It should be noted that for the two months of the experiment, the weight of the juvenile sea cucumbers increased 10.9-fold in the control group (2.47g), 15.2-fold in the experimental group 1 (3.27g), and 2.0-fold in group 2 (2.43g). The data obtained indicate a dose-dependent effect of the introduction of DNA from salmon milt on the growth of body weight of juvenile sea cucumbers.
According to the results of the study conducted, a decrease in the sea cucumber body weight occurred in all the experimental groups by September. The least pronounced body weight decrease was recorded from the animals of the experimental groups.
The above-described experiments were set up under conditions of a constant water temperature of 18±1.0°С in tanks. However, the water salinity measurements conducted in the course of the experiment showed some seasonal fluctuations in this parameter (Figure 1).
According to the data presented in Figure 1, the salinity in June was 28–29‰; in July and August, 30–31‰; and since the early September, it decreased to 23‰. It should be noted that the weight of the experimental animals reduced as the water salinity decreased. The reduction in the sea cucumber weight in September, compared to that in August, was by 36.6 and 51.9% in the experimental groups 1 and 2, respectively, and only by 25% in the control group. An analysis of the results of the experiment shows that the feed formulae tested had a varying effect on the weight of juvenile sea cucumbers.
The introduction of DNA in the feed formula at a dose of 1g per 1kg of feed contributed to an increase in juveniles’ weight by 965%; at the same time, providing the sea cucumbers with the feed containing DNA at 5g per 1kg of feed caused their weight to increase only by 358%. The animals’ weight increase in the case of the control feed formula was 816%.
The estimation of feed efficiency values (Table 2) during the first two months of the experiment showed that the growth rate and the feed conversion efficiency for feed no. 1 are higher than those in the control group. At the same time, a comparison of the efficiency values between the feeds with different DNA contents showed as follows: despite the rate of consumption of feed no. 2 is slightly higher than that of feed no. 1, the conversion efficiency of the former is lower, which resulted in a 1.5-fold lower growth rate of juvenile sea cucumbers and their lower survival rate.
|
Date, parameter |
Feed no. 1 |
Feed no. 2 |
Control |
|
June–August |
|||
|
Survival rate, % |
77 |
73 |
83 |
|
Growth rate (%) |
2.38 |
1.65 |
2.15 |
|
Feed consumption rate (g–1day–1) |
9.51 |
9.84 |
9.39 |
|
Feed conversion efficiency (%) |
0.79 |
0.55 |
0.72 |
|
August and September |
|||
|
Survival rate, % |
70 |
70 |
80 |
|
Growth rate (%) |
1.5 |
0.89 |
1.49 |
|
Feed consumption rate (g–1day–1) |
9.21 |
8.7 |
7.7 |
|
Feed conversion efficiency (%) |
0.61 |
0.3 |
0.61 |
Table 2 Efficiency of feed for juvenile Japanese sea cucumber
The trend of variations in the feed efficiency parameters during the period of seasonal freshening (in August and September) indicates a significant reduction in the growth rate of juvenile sea cucumbers. According to the data in Table 2, the growth rate reduction in the control group of animals is related, to a greater extent, with the rate of feed consumption, whereas in the experimental groups the decrease in feed conversion efficiency is more noticeable. Growth of organisms is accompanied by changes in biochemical parameters of their tissues. Some of the biochemical parameters were determined during forming the stocking material and upon the completion of the experiment (Table 3).
|
Specimen |
Date |
Hexosamine content, % |
Water-soluble protein content, mg/g tissue |
|
initial |
June |
0.1 |
9.8±0.1 |
|
No. 1 |
September |
1.2 |
13.7±0.1 |
|
No. 2 |
1.42 |
27.3±0.2 |
|
|
control |
1.34 |
15.7±0.1 |
Table 3 Level of hexosamines and water-soluble proteins in tissues of juvenile Japanese sea cucumbers
The data in Figure 2 show that all three types of experimental feeds caused a 1.4–2.8-fold increase in the level of water-soluble proteins in tissues of the juvenile sea cucumbers. In this case, the most pronounced effect of increase in water-soluble protein content was recorded for the feed with a high DNA content (no. 2). The difference in efficiency between the feeds with different DNA levels was 2-fold for this parameter. At the same time, the efficiency of the feed with a DNA content of 5g/kg was by 70% higher than that of the control feed. Hexosamines are monomers of glycosaminoglycans which are the main component of the extracellular matrix contributing to the formation of the dense structure of muscular sac in sea cucumbers.
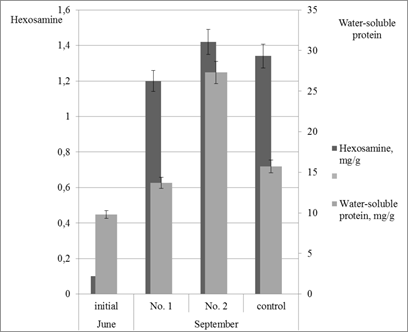
Figure 2 Level of hexosamines and water-soluble proteins in tissues of juvenile Japanese sea cucumbers.
Feeding the juvenile sea cucumbers with the control feed resulted in a 13.4-fold increase in hexosamine content. The experimental formulae with DNA increased the level of hexosamines 12–14 times. Apparently, the use of DNA in feed formulae stimulates the growth of muscle tissue and, as a consequence, the biosynthesis of proteoglycans containing hexosamines.
The intensive development of aquaculture necessitates searching for new efficient components and inventing feed formulae that would ensure production of viable juveniles. Nucleotides perform numerous physiological and biochemical functions such as transfer of chemical energy, participate in biosynthesis, biological regulation, and are coenzymes.7
Numerous studies on different species of aquatic organisms confirm that the introduction of nucleotides in feed increases the growth rate in some fish species.8,9
It was shown that the inclusion of nucleotides has a dose-dependent effect for each fish species: 0.5g/kg of feed for common carp10 1.5g/kg for grouper11 and 2g/kg for rainbow trout.12
According to the conducted studies, the introduction of salmon DNA in feed for juvenile sea cucumber (0.23–0.28g) in an amount of 1g per 1kg of feed contributes to an increase in animals’ weight to a value by 28.7% higher than in the control within the first two months of use. Nevertheless, the feed supplemented with DNA did not influence the survival rate of the juvenile sea cucumbers in the experimental groups, which may also be associated with the negative effect of water freshening. However, the use of DNA-containing feed during this period helps to maintain the rate of consumption of feed and its conversion efficiency.
At the same time, the introduction DNA into the feed in an amount of 5g per 1kg did not affect on young sea cucumber weight. Feeding sea cucumber with the addition of salmon milk DNA for 3 months did not affect the weight of juveniles sea cucumber (1mg/kg), or contributed to weight loss (5mg/kg).
The data obtained are consistent with the study of Wei with co-authors (2015) which showed that inclusion of nucleotides in feed formulae for Japanese sea cucumber (with the initial weight of 5.87±0.03g) showed that the specific growth rate was significantly higher in animals that consumed feed with a nucleotide content of 375mg/kg, as compared to those fed a feed without nucleotides added. In conclusion, it was shown that nucleotides, as a dietary component, actually increase growth rate, nonspecific immunity, and resistance to diseases in Japanese sea cucumber.13
The data obtained indicate the dose-dependent effect of introduction of DNA from salmon milt on the weight increase in juvenile sea cucumber.
In addition, juvenile sea cucumbers are sensitive to water salinity variations due to the high permeability of their external teguments. Our findings are consistent with the earlier studies, according to which the unusual size-frequency distribution of echinoderms in waters with significant salinity fluctuations is probably explained by the lower tolerance of small individuals to hypoosmotic stress. 14,15
The use of DNA from salmon milk in feed formulae in the amount of 1mg/kg, apparently stimulates the growth of muscle tissues and, as a consequence, the biosynthesis of proteoglycans containing hexosamines. Thus, the study conducted by us shows that the inclusion of nucleotides from fish milt in feed formula is promising for on-growing of juvenile Japanese sea cucumbers. The use of DNA extracted from salmon milt may provide a new strategy for controlling health of sea cucumber in aquaculture.
None.
The author declares that there are no conflicts of interest.
None.

©2019 Kovalev, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.