Journal of
eISSN: 2378-3184


Research Article Volume 6 Issue 2
Sulaimani polytechnic university, Iraq
Correspondence: Karzan F Namiq, Sulaimani polytechnic university, Iraq
Received: March 29, 2017 | Published: August 7, 2017
Citation: Namiq KF, Milne D (2017) Effect of Fillet Thickness on Quality and Shelf Life of Gravlax Salmon. J Aquac Mar Biol 6(2): 00149 DOI: 10.15406/jamb.2017.06.00149
Gravlax Atlantic salmon is a raw fish product typically stored at chill temperatures that will go through no further heat processing before consumption. Bacterial spoilage can reduce shelf life while pathogens can represent a serious hazard for susceptible consumers. This experiment was conducted to provide an estimate of the shelf life of 2 thicknesses of gravlax salmon stored at 4°C by monitoring, pH, water activity (aw), salt levels over a 21 day period and microbial numbers (TPC) over 41 days. Total plate counts for both the 1cm and 2cm thicknesses remained at less than 104 CFU/g at the conclusion of the experiment on day 41. Salt levels were 11.41% and 6.85% and water activity values 0.83 and 0.85 for 1 and 2 cm thicknesses respectively. From these figures it was concluded that both the 1 cm and 2 cm thicknesses of gravlax treated Atlantic salmon stored at 4°C have shelf- lives longer than 41 days.
Fish is vital source of animal protein and other elements for healthy body maintenance.1 Fish also provides a good source of high quality protein and contains many minerals and vitamins. It is susceptible to spoilage and quality loss can happen very quickly after catch.2-5
There is an increasing demand for fish and fish products around the world.5 Seafoods have traditionally been popular in many countries as an alternative source of animal protein.6 Nowadays, more people are returning to seafood as a healthy alternative to red meat. The low fat content in many fish species (white fleshed, demersal) has positive impacts on coronary heart disease due to the n-3 polyunsaturated fatty acids. This is particularly important for health of conscious people especially in affluent countries, where heart disease mortality is high.6
Despite many advantages of seafood consumption, it has disadvantages such as causing disease due to microbial infection or intoxication.6 Also, seafood varies from other types of food in many ways, the majority of seafoods are still extracted from a wild population. The processors of wild catch seafood are limited in their choices of raw material to what is available in respect of size, condition and species of fish landed by fishermen.6
Certain pre-packed, ready to eat foods with long shelf life stored at refrigerated food temperatures are of high risk because of Listeria monocytogenes.7,8 These products will be eaten without additional heat treatment and therefore represent a significant risk to selected consumers (young, old, pregnant and immune compromised). Examples for these foods are cold smoked and gravlax (gravid) from salmon and rainbow trout.9
There are many ways to spell and pronounce of gravlax such as gravid laks, gravid lax, graflax.10 All of them related to the Nordic dish of cured raw salmon. Gravlax is translated from the Swedish as buried salmon- grav means grave and lax means salmon that referred to the old custom of salting salmon or other freshly caught fish. After salting fish were left to cure in the ground at temperatures near 0°C.10 Ultra –fresh salmon is clearly important for making gravlax for both food safety reasons and to ensure the best taste and texture (quality).10 Gravlax products are produced by rubbing salmon fillets with a mixture of sugar, salt, pepper and covered with the herb dill and stored at refrigerated temperatures to cure for up to 2 days in plastic bags.9
Salt in the cure acts in three ways, firstly, salt restricts the growth of some species of bacteria and extends the stability and shelf life of salmon. It also reduces water activity by osmosis, creating a denser texture and it tenderizes salmon by breaking down the structure of protein.10
There are many problems concerning the safety of seafood. Seafood is generally made unsafe because of the existence of pathogenic microorganisms, which can take place naturally or can be transmitted to the seafood after it is caught by contamination with people.11 Pathogenic bacteria, which are normally associated with human or warm blooded animals and their faeces, and not normally present in fish or seafood products include Listeria monocytogenes, Salmonella, Escherichia coli and Stapylococcus aureus.12-13 Seafood is more perishable than other high protein products because of the high level of soluble nitrogen compounds in the tissue.14 Activities of microbes are responsible for changes in flavor, odor, texture and color. All sea foods are susceptible to environmental contaminants, including pathogens from harvesting and on board ship handling process.14 Until recently the microbiological safety of seafood was made certain by testing final products at a laboratory.11 Final product testing was very costly and of limited value.11 A new system was developed that relied on the assessment of microbiological hazards related to the products. This system called Hazard Analysis Critical Control Point (HACCP) system, which is being adopted right through the world.11
Shelf life of food can be defined as the maximum length of time a given product is suitable for human consumption.15 In seafood market, fresh and frozen shelf life is a very vital consideration. Knowing the remaining shelf life is very important and allows the processor and retailer to plan the length of time a product can be stored.15 Extended shelf life allows control of the market and reduces waste. By adding to the shelf life allows the market to achieve better economic returns and ensure quality.15 The shelf life of fish could be determined by physical, chemical and microbiological analysis.16
The shelf life of fish products is usually restricted by microbial activities that are affected most importantly by storage temperature.17,18 Typically the shelf life of fish fillets that are kept at chill temperatures ranges between 7 to 14 days (depending on species, harvest location and season).19,20 In Australia chilled seafood competes with other fresh protein products. The customer during purchase relies on sight and smell for giving indications of future eating performance.21 In addition, consumers are progressively demanding more consistently high quality food, and have expectations that such quality will be kept at a high level during the period from production to consumption.22,23
Temperature is the most important factor in determining the shelf life of fish.6 The rate of bacterial spoilage and enzyme breakdown is mediated by temperature.15 Quality of fish is strongly affected by the time and temperature between harvest and consumption as temperature impacts both bacterial growth and autolysis.24 Therefore, time alone will not provide a good indication of freshness and product quality.25,26 Small changes of temperature in the range of 0 to 10 °C have a major influence on microbiological growth.6 This range is also similar to that found in refrigerated storage systems. Most microbes are incapable of growth at temperatures below 5°C but Listeria monocytogenes has been documented growing at chill temperatures of 4°C.
Many traditional preservation methods have been developed using salt and sugar to control microbial growth. Water is necessary for survival and growth of microorganisms.27 The preservation process results from the addition of salt to a food product causing an imbalance and by osmosis, dehydration results.27 The mass transfer mechanism of salt has two major fluxes. First, loss of water in fish products is due to the osmotic phenomena. Second, flow of water from lower salt concentration zones (inside food) to higher salt concentration zones (outside food), dissolving salt.28 In addition salt levels in the flesh correspondingly increase. Salt concentration in the flesh is very important. Elevated salt levels greater than 10% will be sufficient to restrict the growth of clostridium botulinum and the majority of pathogens and spoilage bacteria.
The consequence of adding salt and sugar is a reduction of water activity which is a measure of unbound, free water molecules in food products. The range of water activity is between zero (water absent) and 1.0 (pure water).29 Shelf stability can be determined through water activity unlike water content. It can predict which microorganism will be possible sources of spoilage and infection due to their water requirements.
Sugar is used in gravlax to offset the taste associated with elevated salt levels in the flesh. While sugar is most commonly used to preserve confections it also decreases the amount of free water available (water activity) for growth of microbes Smith and Stratton, 2007. High levels of sugar are effective in preventing the growth of yeasts and molds. Salt is more effective weight for weight than sugar as salt ionizes to a sodium cation and chloride anion attracting a sheath of water molecules. These ionically related molecules of water are unavailable for use by microorganisms and there is a propensity for the ionic forces to dehydrate microbial cells.30
The aim of this study was to estimate the quality of salmon gravlax shelf life at 1 and 2 cm thick of fillets, to note changes in pH (chemical analysis), to observe microbial growth (microbiological analysis: TPC) and to note changes in water activity.
Experimental design
This study sought to establish the shelf life of different thicknesses of Atlantic salmon gravlax stored at a mean temperature of 4°C. Skinless Atlantic salmon fillet portions were allocated to treatments based on mean thicknesses of 1 and 2 cm before treating with salt, sugar and fresh dill. Analytical tests included flesh salt content measured on day 2, pH and water activity (aw) until day 21 and microbiological total plate count (TPC) tests until day 41.
Fish harvesting
Atlantic salmon (Salmo salar) were grown at the aquaculture center at the university of Tasmania and harvested in July 2015 before being transported to the Australian Maritime College (AMC), Beauty Point seafood laboratory. At harvest fish were first treated with a fatal dose of sedative. Unconscious fish were removed from the tanks before receiving a blow to the head as per animal ethics guidelines. Whole fish were repacked before freezing at Beauty Point.
Facility preparation
Before starting fish processing, all surfaces, processing facilities, tools and materials (cutting board, tray, scale, bench and knife), were cleaned by using detergent (Tiger Plus, Applied Chemicals-Forming alkaline, chlorinated detergent). At the conclusion of cleaning, facilities were then disinfected with a commercially obtainable quaternary ammonium compound (FS Formula 7000, Calman Australia) used at a dilution of 500: 1 as suggested in the manufacturer’s instructions
Fish processing
Whole fish were frozen and stored at -25°C until use. In this experiment 10 fish with an average whole weight of 1.1689 kg ± 0.075 were used. Frozen fish were thawed in a chiller set at 4°C for 2 days before weighing, gutting, and filleting. Whole salmon fillets were trimmed of visible lateral fat before the removal of pin-bones and final rinsing. Fillets were allocated to 1 and 2 cm thickness treatments.
Marinating process
Fresh filleted salmon were rinsed gently, and patted mostly dry using paper towel. Salmon fillets were put on a tray, salt, sugar and dill were shaken over the fillets before wrapping in a layer of plastic. Fillets were subsequently further tightly wrapped in at least two more layers of plastic: one around the middle and one lengthwise to prevent leakage and remove air gaps. Wrapped fillets were well mixed with the curing mixture so all surfaces of the fish received an even cure. Final products were vacuum packed (model COMPACK Kramber Greb, Germany). Packed fillets were put in the refrigerator at 4°C and turned over morning and evening for 48 hr. Recipes of the fish, salt and sugar ratios for 1 cm and 2 cm thick gravlax are presented in (Table 1).
|
1 Cm Thick fillet recipe |
2 Cm Thick fillet recipe |
||
|
Ingredients |
Ratio |
Ingredients |
Ratio |
|
Fillet (1cm) |
1000 g |
Fillet (2 cm) |
1000 g |
|
Salt |
200 g |
Salt |
200 g |
|
Sugar |
100 g |
Sugar |
100 g |
|
Fresh dill |
1 bunch |
Fresh dill |
1 bunch |
Table 1 Recipes for 1 and 2 cm fillet thicknesses of gravlax salmon
Microbial analysis
A total aerobic plate count (TPC) method (AOAC, 2000) was used to analyze microbial quality, according to AOAC official methods with some changes. Changes included incubation temperature of 25°C as suggested by the ICMSF for aerobic plate counts (APC) of seafood products (ICMSF, 1986).31 In addition, sample volumes were decreased proportionally because of the capacity of the stomacher. Around 10 gram of product for every treatment was put into the stomacher and weighted. Sterile water (90 mL) was added to the bag and both were stomached for 1.3 minute (Stomacher - Lab Blender 400, London). Serial dilutions for each replicate were prepared in Macartney bottles containing 9 ml of sterile 0.5% peptone water. Pour plates were prepared with 1 ml for each dilution transferred to 900 mm petri dishes.
Plate Count Agar was prepared according to the manufactures instructions and was tempered in a water bath at 48°C. All pour plates were incubated at 25 °C for 48 hours or until recognizable colonies could be identified. Some days, incubation periods were extended to 120 hours due to the colonies slow growth. Means of 2 replicates are shown as colony-forming units for gram of muscle (CFU/g).
Water activity (aw)
Water activity was determined by using Novasina aw- center, which was calibrated at 25 °C by using sodium chloride (water activity 75.3) and magnesium chloride (32.5 water activity) before placing samples into it. 10 g of gravlax was ground in a mortar and pestle until a homogenous mixture were achieved. 5 g of samples (gravlax) were placed in capsules until readings stabilized with four arrow bars recorded for each sample.
Salts
Salt content tests were done on day 2 of the experiment using AOAC standard method 937.09,32 after maturing of gravlax for 48 hours. Gravlax products were taken out of the cold room (4 °C) and rinsed with water to remove salts from both groups. 10 g sample was ground with a mortar and pestle until a homogenous mixture was achieved. 2 g samples were weighed into flasks by using an analytical balance (HF-300 G) and 50 mL of standardized AgNO3 (0.1006 M) added to every flask. Subsequently 10 mL of aliquot of HNO3 was added to the samples and heated on a hot plate in the fume hood until digestions of samples were completed. Samples were cooled down for 15 minutes prior to add 50 mL of deionised water to both flasks. Finally a 3 mL aliquot of FeNH4 (SO4)2 12H2O indicator was added to every flask and titrated (0.1 M NH4SCN) until a brown color change was noted. Values of salt content were calculated to obtain a mean salt content of the gravlax.
Calculation used:
pH measurement
The pH for each sample was taken and measured by using a pH meter (WTW PH 330, Weilheim Germany) in arrangement with internal (WTW Sentix SP) electrode. The pH meter was calibrated using buffers at 7.0 and 4.0 before use. pH readings of flesh were taken by entirely inserting the glass tip of the WTW Sentix SP electrode into the flesh as per the method of Thomas.33
Statistical analyses
Statistical analysis for this experiment was conducted by using SPSS 22 for windows. All graphs in this experiment were presented as mean values ± standards error mean (SEM).Differences between means were assumed to be significant at P<0.05.
Microbial analyses
Total aerobic plate counts (TPC) were conducted on days 0, 2, 5, 8, 12, 15, 18, 21, 32, 41 and presented in (Figure 1). The TPC for raw material on day 0 for both thin and thick treatments (1 and 2 cm thick fillets) was a mean (n=2) of 500 and 600 (CFU/g), respectively. On day 2 after curing mean microbial loads were 420 and 630 (CFU/g). Counts increased slowly and by day 21 microbial loads had increased to 960 for 1 cm thick and 1300 (CFU/g) for 2 cm thick treatments. On day 41 at the end of monitoring microbial loads had increased to 1380 for 1 cm thick and 3497 (CFU/g) for 2 cm thick gravlax fillets.
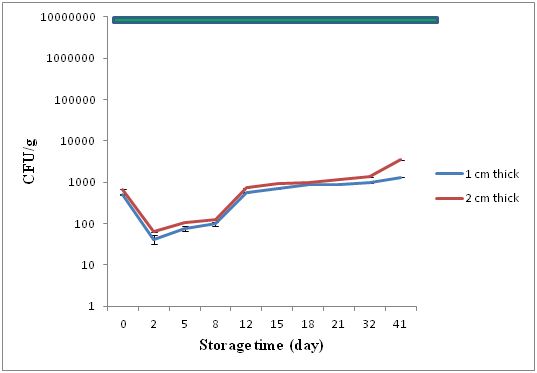
Figure 1 Mean TPC (± SEM) for 1 and 2 cm thick fillets sampled on 0, 2, 8, 15, 21, 32 and 41 days, ICMSF (1986) standard of 107CFU/g is indicated by the green line.
Water activity
Water activity (aw) values were measured on days 0, 5, 8, 12, 15, 18 and 21 and are presented in (Figure 2). The values of aw on day 0 (raw fillets) were 0.911± 0.002 for 1 cm thick fillets and 0.92 ± 0.0021 for 2 cm thick fillets. On day 5 after treatment aw values for both treatments had decreased to a mean of 0.83 and 0.85 for the1 cm thick and 2 cm thick fillets. Water activity subsequently increased for the rest of the experiment, reaching 0.849 ± 0.0015 for 1 cm thick and 0.859 ± 0.0010 for 2 cm thick on day 21.
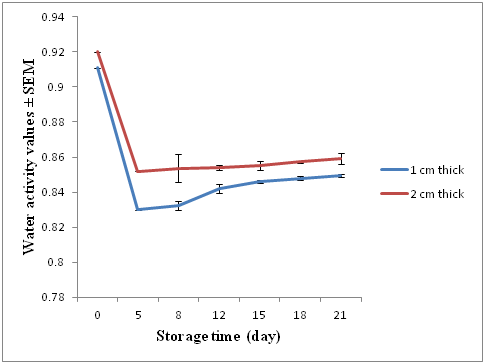
Figure 2 Mean water activity (aw) (± SEM) for 1 and 2 cm thick fillets sampled on 0, 2, 8, 15 and 21 days.
Salt
Salt content measurements were done on day 2 of the experiment after rinsing residual salt from the treatments with fresh water and drying with absorbent paper. 3 replicates for each fillet thickness were measured, the mean calculated and shown in (Figure 3). Salt content for each treatment is presented as a percentage. The percentage of salt in the 1 cm thick fillets was 11.41% (± 1.45) and approximately double that of the 2 cm thick fillets at 6.85% (± 0.40).
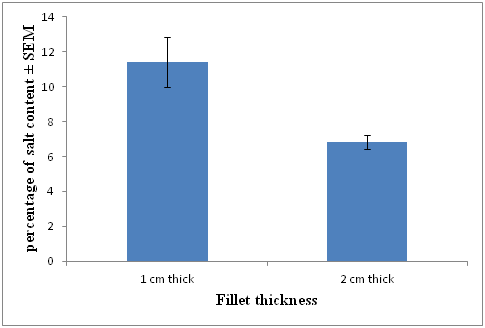
Figure 3 Mean (n= 3) salt content (± SEM) of 1 and 2 cm thick fillets sampled on day 2 of the experiment.
pH
pH was measured on days 0, 2, 8, 15 and 21 of the experiment. The day 0 pH of raw fillets before curing was 6.23 ± 0.005 for 1 cm thick fillets and 6.35 ± 0.06 for 2 cm thick fillets. On day 2 pH values had decreased for both treatments reaching 5.65 ± 0.055 and 5.83 ± 0.055 for 1 and 2 cm thick fillets respectively. From day 2 until day 21 pH values increased slightly and reached 5.7 ± 0.005 for 1 cm thick and 5.87 ± 0.03 for 2 cm thick at the conclusion of the experiment. There was a significant difference in pH between the 2 different thicknesses of gravlax fillets (Figure 4).
In general, shelf life is quoted in terms of storage time in days before sensory rejection. Associated with sensory rejection are microbial and chemical parameters that individually or in combination provide indicators to spoilage.19,20 In Australia, there is no indicative microbial standard for the shelf life of chilled seafood but the maximum safe limits for Total Plate Counts (TPC) are generally agreed at between 5 x 106 CFU/g (AQIS) to 107 CFU/g (ICMSF).
Initial microbial loads after processing were low and indicate fresh fish and sanitary processing conditions. After curing with salt and sugar microbiological loads in both thin and thick fillets remained close to initial counts. Between days 2 and 30 microbial counts for both treatments remained similar before counts of the thin fillets exhibited accelerated growth after day 30. The lack of difference in microbial growth between the two treatments can related to the salt and sugar contents in both.
In microbial terms both 1 cm and 2 cm thick fillets exhibited shelf-lives in excess of 41days at which time counts for both treatments were less than 104 CFU/g. These numbers are significantly less than the 106 (AQIS) and 107 (ICMSF) maximum safe limits recommended for chilled fish products. The basis for this extended shelf life at a storage temperature of 4°C may be attributed to the salt levels in the flesh and associated aw of the gravlax fish. The ratio of 10:2:1 fish to salt and sugar at the brine time of 48 hr resulted in residual salt levels of 6.85% and 11.41% and water activities of 0.85 and 0.83 respectively for 1 cm and 2 cm thick fillets.
Table 2 lists growth factors for a range of typical spoilage, pathogenic bacteria, molds and yeasts. In summary the combination of storage temperature, salt and water activity of the gravlax Atlantic salmon would prevent the growth of all organisms listed in Table2.
|
Spoilage |
Salt (%) |
aw |
Temp °C |
pH |
|||
|
Pseudomonas spp. |
0-10 |
0.94-0.95 |
15- 40 |
4-9 |
|||
|
Pathogen |
|||||||
|
Staphylococcus aureus |
0-25 |
0.83-0.99 |
7- 47.8 |
4.5-9.3 |
|||
|
|
Other |
||||||
|
Mold |
0.7-0.80 |
10-35 |
2-9 |
||||
Table 2 Growth factors for spoilage, pathogen bacteria and other (mold and yeast) (Ratkowsky et al. 38 Food and Drug Administration.42
The gravlax water activity is sufficiently low to prevent the growth of all listed bacteria both spoilage and pathogenic with molds being the only organism capable of surviving at the measured levels for both thicknesses.
Residual salt levels were sufficient in the thin fillets to eliminate all spoilage organisms excluding Pseudomonas spp. and Lactic acid bacteria. Only Listeria monocytogenes a pathogen would be capable of growth at the residual salt levels. For the thick fillets salt levels were either sufficient or at the margins for growth of nearly all bacterial organisms excluding Listeria monocytogenesand Staphylococcus aureus.
Storage temperature was sufficient to prevent the growth of all organisms except Listeria monocytogenes. The inhibitory effects of the product and storage parameters should prevent the growth of all the listed organisms and explain the long lag phase observed for both thicknesses. At day 41 microbial growth was only log 2 above initial counts indicating additional time would be required to reach maximum numbers for product safety.
Salting of fish products decreases water activity (aw), therefore, it prevents growth many of spoilage microorganisms.34,35 In addition, the chloride ion is toxic for many microorganisms.36
There was a significant difference between the salt concentrations of thin and thick fillets 6.85% ± 0.40 and 11.41% ±1.44 respectively. The difference in salt uptake between thin and thick fillets is confirmed by FAO,37 which reported that thick filleted fish tend to absorb salt slower than thin fillets. The relative rate of salt uptake also decreases with fillet thickness with the uptake slowing towards the center of fillets. Piggot and Tucker38 also reported factors that affect salt absorption include: grain size, purity of salt and the thickness of the flesh.
The salt levels recorded in the thick gravlax product are at the upper end of that in which most microbial organisms will grow.6 This combined with the low water activity and chill storage temperatures while sub lethal to many bacteria would in combination result in metabolic stress and an inability to grow.39 In thin fillets the salt levels measured would preclude the growth of all but halotolerant bacteria including Staphylococcus aureus and Listeria monocytogenes.
In general bacteria are more sensitive than molds and yeasts to reduced water activity and approximately all are prevented from growth at water activities less than 0.90 to 0.91 while molds and yeasts grow at 0.7-0.80 and 0.87-0.94 respectively.40
Initial water activity (aw) values in frozen defrosted raw flesh were 0.91 and 0.92. These values were lower than typical water activities observed in fresh flesh of 0.95 to 0.99 (Fraser, 1998). These values may be influenced by the high oil content and slow freezing of the raw material that was used for the gravlax. Slow freezing is associated with post freezing driploss while high oil levels in the salmon flesh result in lower water activities.6
Reductions in aw values are the result of osmotic imbalance due to the addition of salt and sugar in the brine. Water flows from low salt concentration to high concentration zones resulting in less water in the fish flesh.28 Water activity values after curing were significantly lower at 0.83 and 0.85 in thin and thick fillets, respectively. These values were in the range 0.75 to 0.85 reported for cured fish products by Piggot and Tucker.38
pH in this study was used to confirm microbial total plate count numbers. Generally increases in microbial numbers are associated with increases in pH values. In this study the small increases in pH over time confirms the relatively small increases in microbial counts.
Initial pH values for both fillets (thin and thick) were 6.23 and 6.35, respectively. These values were in agreement with Asli et al.,41 who noted the pH value of flesh salmon as 6.46 before any treatment. In day 2 of experiment (after curing) pH values were significantly decreased in both fillets thicknesses, 5.65 thin and 5.83 thick. Finally, the low pH values impact the water-holding ability of the muscle, resulting in increased exudate loss that is related to as driploss.42-50
In this experiment gravlax salmon were stored for 41 day, during in this period microbial loads did not exceed 104 CFU/g and was well below the indicative standard of 107 CFU/g (ICMSF). From these figures it may be extrapolated that the shelf life of gravlax should be longer than 45 days. It could be expected that the thin variant would have a longer shelf life than the thick due to the lower water activity and higher salt content. This product had much longer shelf life than fresh fillet of salmon, which is 15 days at 4 oC, while gravlax had 45 days shelf live at 4 oC. Longer shelf live would help waste reduction of gravlax due to spoilage, can be stored for long time at stores and long chain of distribution.
None.
None.

©2017 Namiq, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.