Journal of
eISSN: 2378-3184


Research Article Volume 5 Issue 4
1Coldwater Fishes Research Center (CFRC), Lead Center of Networking of Aquaculture Centers in Asia, Iran
2Iranian Fisheries Research Organization (IFRO), Iran
Correspondence: Hossein Abdollhay, Iranian Fisheries Research Organization (IFRO), Iran, Tel 98-21- 66943860
Received: December 12, 2016 | Published: April 4, 2017
Citation: Bahramian B, Abdollhay H, Lashgari SN (2017) Determination of Suitable Weight of Caspian Trout, Salmo Trutta Caspius Kessler, for Sea Ranching Purposes. J Aquac Mar Biol 5(4): 00125 DOI: 10.15406/jamb.2017.05.00125
This study investigated the changes of osmoregulatory ability of different size of Caspian trout (Salmo truuta caspius kesller), Salmo trutta released from freshwater to 13 ppt seawater. Authors concluded that the 20g fish with 12 cm is suitable for release into seawater for sea ranching purpose.
This study was implemented to determine the smolt weight of Caspian trout, Salmo truuta caspius kesller, for sea ranching purposes. In this survey, the osmoregulatory of the experimental juveniles (including 3, 5, 10, 15, or 20 g groups) were followed by investigation the concentration of serum sodium of the fish blood in seawater and the time-course of changes in serum sodium concentration was followed after direct transfer to seawater containers (13 ppt in salinity).
According the results, the concentrations of the serum sodium were 137-144 meq/l in juveniles in freshwater, and increased slightly after 3 hours of direct transfer to seawater simultaneously (149-156 meq/l). After 7-13 hours, it was measured in a maximum level (152-158 meq/l), and then attained seawater-acclimated level after 24 h (148-154 meq/l). When acclimated juveniles in seawater were transferred back into the freshwater, the concentrations of serum sodium reduced in all of the weight groups after 48 h (142-147 meq/l), but were slightly higher than those in freshwater before direct transfer into seawater containers. Serum sodium concentration increased slightly in the juvenile with weight of 20 g (153-154 meq/l); therefore, better osmoregulatory were detected in juvenile of 20 weight g, than the others. Also, the chloride secretory cells were not detectable in their freshwater stage, and appeared only after transfer to seawater. In addition, it seems the chloride secretory cells in juvenile with weight of 20 g, were better adjusted than the other weight groups after transfer to the seawater.
In this study, behaviour migratory changes of Caspian trout juveniles were evaluated by tagging method (cutting the adipose fin) after releasing into Tonekabon river of Caspian sea. The three stations in the river were selected (including: estuary, 250 m to the estuary and 500 m to the estuary) to evaluate behaviour migratories. The juveniles with weight of 20 g were found at the estuary zone more than the other zones. Tendency to leave river toward the sea in compare of others. So, this is the recommended weight of Caspian trout as smolt size and could be used for releasing operations into the rivers of Caspian Sea.
Keywords:Caspian trout, Salmo trutta caspius Kessler, Smolt, Osmoregulation, Serum sodium, Freshwater, Sea water, Behaviour migratory, Sea ranching, Iran
Anadromous salmonids undertake a metamorphosis, the parr-smolt transformation, as they prepare for migration to the sea. Photoperiod, induced changes in physiology, body shape, and behavior transform the cryptic, bottom-oriented resident form, or parr, to the migratory, scholling form.1-7
This metamorphosis is termed smoltification and the resulting migrant is termed a smolt. The process of smoltification is a major life history event, with fundamental changes in body form. Timing and duration of the metamorphosis and downstream migration to the sea is determined by a species-specific, genetically-determined life history pattern and environmental events governing growth rate and size. For example, chum and pink salmon smoltify almost immediately upon absorption of their yolk sacs and swim up in the late winter or early spring.8,9
Chinook, Coho, Sockeye, and steelhead normally smolt as yearlings or two year-olds during the period of increasing day length in late spring (Houston, 1961).4 Depending on life history type (stream or ocean form), or the size attained in their first year of life, some chinook may smoltify as yearling or sub-yearlings.
Caspian trout (Salmo trutta caspius kessler) is the most expensive and luxury sea food in the south of Caspian sea. It has a rare and limited distributions in the coastal sea and rivers of the southern of Caspian sea. For this reason, Shaheed Bahonar rearing and breeding center of salmonids fishes in Kelardasht-Iran, releases 300 × 103 to 400 × 103 smolts of Caspian trout in many rivers of the region every year.
Teleosts are hypoosmotic in seawater and balance the influx of ions and the efflux of water by excreting excess monovalent ions in the gills, absorbing fluid from ingested seawater in the intestine, and excreting excess divalent ions via the kidney. The key enzyme to transport processes in the gill and intestine is the membrane-spanning protein Na+,K+-ATPase. In fish, gills play a very important role in regulating serum salt concentrations and transporting ions between the blood and the seawater. Unique cells, called chloride cells in the gills, are specialized in transporting sodium, potassium, and chloride. These cells have large concentrations of Na+,K+-ATPase channels and hence play a major role in regulating serum sodium/potassium levels in fish.10-13
The present study was carried out to examine the changes in osmoregulatory ability of the juvenile during growth and prolonged rearing in freshwater, by following the changes in serum sodium concentration after transfer from freshwater to seawater or vice versa. Also, behaviour migratory changes were evaluated by tagging method (cutting the adipose fin) at three zones of Tonekabon river (including: the estuary, 250 m to the estuary-releasing point- and 500 m to the estuary). The purpose of this study was to determine the smolt weight of Caspian trout (Salmo trutta caspius) for releasing into the selected rivers of the south of Caspian sea in Iran.
All of the experimental fish juveniles (Salmo trutta caspius) were reared in freshwater tanks in Shaheed Bahonar hatchery center. The seawater (13 ppt in salinity) was transported by a tanker from Caspian sea shore to Shaheed Bahonar hatchery center and it was kept in the fiberglass tanks equipped with an electrical air injection system. About 2000 of the juveniles were randomized five different weight groups (Table 1) and kept in 5 freshwater tanks. The blood of 60 samples of these juveniles in different groups were collected by cut of caudal vein and their serum sodium concentrations were examined by flame photometer. Then, the rest of the juveniles in all groups transferred from freshwater tanks into the similar fish tanks with 13 ppt seawater to evaluate subsequent changes of serum sodium concentrations of the juveniles for 24 hours at intervals of 3, 7, 13, or 24 h. Then, about 60 juveniles in every group were transferred back to freshwater tanks, and serum sodium concentrations were examined after 48 h (twice tests for each group).
|
Group |
Number |
Weight (gram) |
Length (cm) |
|
1 |
400 |
3±1 |
5.5±2 |
|
2 |
400 |
5±1 |
7.0±1 |
|
3 |
400 |
10±2 |
9.8±2 |
|
4 |
400 |
15±2 |
11±1 |
|
5 |
400 |
20±2 |
12.2±1 |
Table 1 The number of the juveniles in different weight groups, which were used for serum sodium concentration examination
For evaluate the behaviour migratory changes of Caspian trout, Salmo trutta caspius, juveniles, about 10020 juvenile from available total juvenile numbers (66500 fish), which kepts in freshwater (with different group weights of 3, 5, 10, 15, 20 & >25 g and number of 1670 fish) were selected and tagged (by cutting the adipose fin) (Table 2). Then, they were released into Tonekabon river just in three considered stations (Including: Station 1-Estuary Zone: in the estuary zone, Station 2-Releasing zone of juveniles to the river: 250 m to the estuary and Station 3-Underbridg zone: 500 m to the estuary) in the river. For this propose, the juvenile were recatched by a cast net (with 1.5 meters opening and 6 mm mesh size) in four attempts at the three mentioned stations.
|
Group |
Number |
Weight (gram) |
Length (cm) |
|
1 |
1670 |
3±1 |
5.4±1 |
|
2 |
1670 |
5±2 |
7.1±2 |
|
3 |
1670 |
10±2 |
9.8±2 |
|
4 |
1670 |
15±2 |
10.9±1 |
|
5 |
1670 |
20±1 |
12.1±2 |
|
6 |
1670 |
>20 |
>13 |
Table 2 The number of the labled juveniles in different weight groups, which were released in Tonekabon River to evaluata their behaviour migratory changes
The data were statistically analyzed by one-way analysis of variance (ANOVA). The differences were considered significant, when p < 0.05.
The seawater challenge test indicated that serum sodium concentrations increased in all of the weight groups when the juveniles transfer from freshwater tanks to seawater containers. There were significant increasing in serum sodium concentrations after 3 h and 13 h of direct transfer to seawater; while these concentrations were decrease in 7 or 24 h of direct transfer to seawater. Serum sodium concentration decreased in all of the weight groups when juveniles were transfer back into freshwater tanks after 48 h; but were still higher than those in freshwater before direct transfer into seawater containers. Serum sodium concentration changes were in the juvenile groups with weight of 20 g than those in the other weight groups (p < 0.05) (Figure 1).
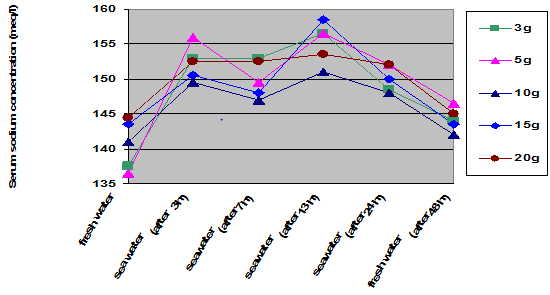
Figure 1 Time-course changes in serum sodium concentration after direct transfer of Caspian salmon juveniles to seawater.
Behaviour migratory changes of Salmo trutta caspius juveniles were followed after releasing into Tonekabon river. In Station 1, the percentage rates of recatched juveniles with weight of 20 g were more than other stations (p < 0.05) (Figures 2-4). Also, at the same station, the percentage of the recatch rate of the tagged juveniles with weight of 20 g, ratio of the total recatched fish in the same weight showed more significant values than the other weight groups (p < 0.05) (Figure 5).
The Caspian trout, Salmo trutta caspius, used in this study showed a relatively good tolerance in salinity changes during different stages of life development periods in weights of 3, 5, 10, 15, 20 and more than 20 g. In the present survey, the time-course of changes in serum sodium concentration of Caspian trout was evaluated in salinity of 13 ppt (the salinity of Caspian sea). When Caspian trout juveniles were transferred directly into the seawater (salinity of 13 ppt), serum sodium concentration of the blood increased significantly after 3 hours simultaneously (149-156 meq/l) and after 7-13 h, it was reached in a maximum level (152-158 meq/l), and then attained seawater-acclimated level after 24 h (148-154 meq/l). The juveniles of 20 g were able to adjust serum sodium concentration within 12 h without showing any sharp peak rate. However, serum sodium concentration changes of smaller juveniles weighing 3, 5, 10, 15 g increased significantly after 3 h, and then decreased after 7 h, and again increased after 13 h of the stay in seawater. These results indicates better adaptability characteristics in seawater of the juveniles weighing 20 g, than the other groups. According to the results, it seems that the osmoregulatory ability of the juveniles weighing 20 g is better than the other weight groups. These changes allow these juveniles to successfully adapt for the osmoregulatory challenges of moving from river to sea. Appropriate timing of entry into the seawater results in reduce death or growth retardation.14 The gills of teleost fish play an important role in ion regulation.15,16,10 Recent studies on fin fishes have documented the changes in a number of hormones, during the parr-smolt transformation.11,12,13 Although the regulation of gill Na+, K+-ATPase is now well understood, a large gap in our knowledge concerns how ion and water absorption by the intestine are regulated by smolt-related hormones.11,12
In addition, the present results indicated that the juveniles of 20 g (average size of 121 mm) were recatched more in the estuary zone at the Station 1, than others. It seems that the juveniles of 20 g (average size of 121 mm), have more tendency to migrate to the seaward `than the other groups. Similar results have been concluded at the base of investigations on smoltilification changes of Atlantic salmon, brown trout (Salmo trutta) (110-200 mm), Masu salmon (Oncorhynchus masou) (110-130 mm), Chinuk salmon – "stream type" (75-125 mm), Steelhead trout (Oncorhynchus mykiss) (125-225 mm).17-21
Therfore, it is suggested that Caspian trout juveniles of 20 g (average size of 121 mm) are better smolt weight for releasing into the southern Caspian sea rivers in Iran.
In conclusion, it is recommended that gradual acclimation in juveniles weighing 20 g was more effective than other groups in stimulating the osmoregulatory mechanisms. Future studies are needed to understand the combined effects of acclimation methods and salinity on the osmoregulatory organs of Salmo trutta caspius. Moreover, interactions from other physiological factors, water qualities, and stresses should also be taken into considerations.
The authors would like to thank Mr. Ali Farzanfar for their expert assistance.
None.

©2017 Bahramian, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.