Journal of
eISSN: 2378-3184


Research Article Volume 12 Issue 2
Division of Biological Sciences College of Arts and Sciences, University of the Philippines Visayas, Philippines
Correspondence: Ebonia B Seraspe, Division of Biological Sciences College of Arts and Sciences University of the Philippines Visayas, Miagao, Iloilo 5023 Philippines
Received: June 06, 2023 | Published: June 29, 2023
Citation: Seraspe EB. Cyclic adenosine 3’5’monophosphate (cAMP) regulates meiotic maturation in goldfish oocytes Carassius auratus (Teleostei: Cyprinidae). J Aquac Mar Biol. 2023;12(2):170-175. DOI: 10.15406/jamb.2023.12.00370
A good understanding of the mechanism of oocyte development and maturation, and the regulatory factors involved therein are important for the success of a breeding program especially of species used in aquaculture. It is crucial in a breeding program for females to supply high quality mature oocytes, which is generally achieved by fine-tuning oocyte meiotic arrest and resumption progression. Therefore, knowledge of the oocyte maturation process is fundamental for the development of methodologies to increase the success of fish production in aquaculture. The regulations of oocyte maturation still are not totally understood. The hypothesis whether elevated levels or a drop in intraoocyte cAMP triggers resumption of meiotic maturation was investigated in denuded goldfish oocytes. In the presence of agents that elevate cAMP like forskolin, dibutyryl cAMP, isobutyl methylxanthine, maturation of denuded oocytes was inhibited. Whereas 2’deoxyadenosine-3-monophosphate, an inhibitor of adenylate cyclase promoted oocyte maturation. Forskolin decreased spontaneous maturation as well as hormone-induced maturation of goldfish oocytes. At 10 µM forskolin added up to 4 h after hormonal stimulation completely inhibited oocyte maturation. Beyond 4 h, however, when oocyte maturation had already started, forskolin can no longer inhibit maturation. Intraoocyte cAMP assayed in denuded oocytes with or without hormonal stimulation decreased immediately within 30 minutes. With forskolin, it did not cause a significant decrease in cAMP not until at 4 h. The results were consistent with the findings that elevated levels of cAMP maintain meiotic arrest while a decrease in cAMP is necessary and sufficient to trigger resumption of meiotic maturation.
Keywords: oocyte maturation, cyclic AMP, meiotic maturation, goldfish oocytes
cAMP, Cyclic adenosine monophosphate; GVBD, germinal vesicle breakdown; MIS, maturation-inducing steroids; MPF, maturation promoting factor; RIA, radioimmunoassay; FK, forskolin; PDE, phosphordiesterase;
During the course of maturation, oocytes undergo morphological changes in which oocyte nuclear envelope breakdown [germinal vesicle breakdown (GVBD)] and usually associated with progression of the meiotic cell cycle.1 Fully grown oocytes are arrested in the diplotene stage of the first meiotic prophase referred to as the prophase I arrest. Hormones such as progesterone and other maturation-inducing steroids (MIS) stimulate the release from prophase I arrest and the resumption of meiosis. A complex cascade of biochemical reactions occurs which leads to germinal vesicle breakdown (GVBD), and thus, the completion of final oocyte maturation.
Uncovering the signals involved in controlling the resumption of oocyte meiosis has been at the forefront of oocyte research for decades. Many of the long-standing controversies in relation to the regulation of oocyte meiotic maturation may seem complete, yet there are still many key questions remain to be resolved.2–4 The mechanism of final oocyte maturation is not well studied in fish as compared to mammals and amphibians. Fish oocytes can be a useful model for studying the mechanism of oocyte maturation. If the mechanism of oocyte maturation is well understood, seed production especially of species used in aquaculture can be easily manipulated. Further study of fish oocyte maturation will lead to the understanding how a signal is transduced to result in a cellular response.
Oocyte maturation and ovulation are regulated by the hypothalamus-pituitary-gonadal axis.5 Final oocyte maturation in most animals is controlled by gonadotropin secreted by the pituitary gland. Gonadotropin induces the follicle cells to secrete a maturation-inducing steroid, which in turn acts on the oocyte to initiate maturation. Different species have different MIS. In amphibians and mammals, progesterone appears to be the natural MIS. In fish, most of the steroids found to be effective are the progestins. Studies done on fish showed that 17α, 20ß-dihydroxy-4- pregnen-3-one (17, 20 P) has been the most potent steroid to induce final oocyte maturation.6–8
The MIS is required at the initiation phase to trigger GVBD. It is no longer necessary for subsequent cascade of events in final maturation. At the initiation phase, MIS interacts with the receptor in the oocyte surface. The signal generated is transduced across the plasma membrane into the cytoplasm, where it elicits biochemical reactions leading to the activation of maturation promoting factor (MPF).9
The second messenger that integrates the intracellular cascade of events which leads to the resumption of meiosis is cAMP. There are several lines of evidence which show that cyclic AMP plays a fundamental role in oocyte maturation.2,4,10–12 The hypothesis whether elevated levels or a drop in intraoocyte cAMP triggers resumption of meiotic maturation was investigated in this study using denuded goldfish oocytes.
Materials
Medium 199, fungi zone, penicillin-streptomycin and L-glutamine were obtained from Gibco Laboratories, Grand Island, N.Y. The labeled antigen, succinyl cAMP-[125I]-tyrosine methyl ester was purchased from Amersham International, Amersham, U.K. The hormone 17α, 20ß-dihydroxy-4-pregnen-3-one and other chemicals were obtained from Sigma except forskolin which was purchased from CALBIOCHEM. Dye reagent for Bradford protein assay was obtained from Bio-Rad Lab, Richmond, California.
Oocyte collection and culture
Gravid goldfish purchased from local fish farm were chosen for experiments by sucking out some ovarian oocytes with a plastic cannula inserted through the ovipore.
Only fish containing oocytes at the tertiary yolk stage (0.8mm in diameter) with central germinal vesicles were decapitated. The ovaries were removed and placed in ice-cold isolation medium, pH 7.2, consisting of Medium 199, 20 mm Hepes, 8 mm NaHCO3, 2 mm L- glutamine, 100 IU/ml penicillin, 100 µg/ml streptomycin, and 1.25 µg/ml fungizone. Small pieces of the ovary were pre incubated for 30-45 min in a Ca/Mg-free medium prepared as described by Greeley et al.,13 except that gentamycin was replaced with 100 IU/ml penicillin, 100 µg/ml streptomycin, 1.25 µg/ml fungizone and 2 mm L-glutamine, to loosen the follicular envelope surrounding the oocyte. Then, the ovarian fragments were transferred to ice-cold isolation medium. The oocytes were isolated and removed of their follicular wall with a finely tipped forceps manually.
Groups of 10 oocytes were incubated in multiwell tissue culture dish (NUNC Inter Med, Denmark) containing 1 ml of incubation medium with the same formulation as the isolation medium except that Hepes was replaced with 30 mm NaHCO3. All cultures were placed in an incubator with humidified atmosphere of 5% CO2, 95% air at 25 oC. After culture, the oocytes were scored for germinal vesicle breakdown, an indicator that the oocyte had undergone maturation. GVBD was considered to have occurred when the germinal vesicle could not be detected after treatment of the oocytes with the clearing agent of glacial acetic acid and absolute ethanol in a 1:1 ratio. Viability of the oocytes was ascertained before the GVBD scoring with trypan blue exclusion test.14
The osmotic pressure of the media used was determined with WESCOR 5500 vapor pressure osmometer and adjusted with NaCl to that of the ovarian fluid when necessary.
Effect of chemical agents that elevate oocyte cAMP
Chemical agents that can change oocyte cAMP levels were evaluated by their effects on maturation, that is, if indeed elevated levels of cAMP will maintain meiotic prophase arrest in goldfish oocytes. The chemical agents used were, forskolin, dbcAMP, and IBMX. Denuded gold fish oocytes were cultured at different concentrations ranging from 0 to 10 µm forskolin, 0 to 10 m, dbcAMP and 0 to 10 m, IBMX with MIS stimulation. Maturation was scored after a 24h culture period. The effect was determined until the time period at which an elevated level of cAMP could inhibit oocyte maturation in a time-course experiment.
All experiments had not less than three replicates and were repeated three times. Results were expressed as mean + SEM (standard error of the mean) of three experiments.
Effect of agent that lower oocyte cAMP
Percent maturation of denuded goldfish oocytes was observed in the presence of an agent that can lower oocyte cAMP. The agent used was 2’-deoxyadenosine-3’-monophosphate, an inhibitor of adenylate cyclase at different concentrations from 0 to 1 mm. Maturation was scored after a 24h culture period in an experiment with three replicates each concentration and repeated three times. Results were expressed as mean + SEM of three experiments.
Cyclic AMP assay experiments
Denuded goldfish oocytes were cultured with or without free follicular layers as well as in the presence of MIS with or without forskolin and in forskolin only. Intraoocyte cAMP levels in these cultures were assayed at 0, 0.5, 1, 4, 8, 16 and 20h in vitro. The culture medium was also assayed for cAMP at the same hour intervals to ensure that differences in the intraoocyte cAMP levels were not due to extrusion of cAMP into the medium. All the experimental treatments were in three replicates and repeated twice.
Cyclic AMP extraction and assay
At the end of cAMP assay experiments, both the media and the oocytes were assayed for cAMP. Denuded oocytes were transferred into 12x75 mm glass tubes containing 0.5 ml of 1 mm theophylline, washed twice with the same solution, boiled for 5 min and stored at -60 oC until use. Likewise, the culture medium was transferred into glass tubes containing 100 µl of 10 mm theophyllline, boiled for 3 min and stored at -60 oC or assayed directly for cAMP without further extraction.
Only the oocytes were subjected to extraction in ethanol to a final concentration of 80%. They were homogenized in a glass tube in ice with a motor-driven Teflon pestle for 2 min. The homogenates were left to settle overnight at 4 oC to precipitate the protein and then, centrifuged at 3000 rpm for 15 min. The supernatant was decanted and evaporated to dryness in a vacuum oven at 55 oC. The extract was redissolved in 0.05 M sodium acetate, pH 6.2. The pellets were stored for protein assay. Cyclic AMP in the oocyte was expressed in terms of mg protein.
Cyclic AMP was measured by radioimmunoassay (RIA) as previously described.15 The labeled antigen was succinyl tyrosine-[125I]-methyl ester derivative of cAMP. The standard curve ranged from 7.8 to 500 femtomole (fm). The sensitivity of the standard curve defined as the dose corresponding to the mean cpm bound at zero dose minus 2SD of the mean cpm bound at zero dose16 was calculated to be 2.14 fm. The intra- and inter-assay coefficients of variation were 8.3% and 9.1%, respectively. RIA data were analyzed using the W.H.O. immunoassay data processing program developed by P.R. Edwards.17 Recovery of the tracer added to the sample was 92%.
Protein assay
The pellets remaining after centrifugation of homogenized samples were dissolved in 0.01% sodium dodecyl sulfate in 1N NaOH. They were boiled and assayed for protein following the Bio-Rad microassay procedure.
Statistical analysis
The meiotic maturation data as well as the cAMP data were analyzed by analysis of variance test (ANOVA) followed by Duncan’s multiple range test using the SPSS software computer program to test differences between treatments. Differences of P<0.05 were considered to be statistically significant.
Effect of chemical agents that elevate oocyte cAMP
Elevation of intraoocyte cAMP by agents such as forskolin, dbcAMP and IBMX inhibited MIS- induced maturation of denuded goldfish oocytes (Figure 1). This inhibitory effect was dose- dependent. Complete inhibition of MIS-stimulated denuded goldfish oocytes was observed at 1 and 10 µM forskolin (Figure 1a), 10 mM dbcAMP (Figure 1b) and 10 mM IBMX (Figure 1c). Even at very low concentrations, 0.001 µM forskolin or 0.01 mM dbcAMP or IBMX, significantly decreased (P<0.05) maturation of MIS-stimulated denuded goldfish oocytes. Forskolin had about 1000x greater inhibitory effect than dbcAMP or IBMX. The half-maximal inhibitory dose of IBMX or dbcAMP was 0.1 mM as contrasted to 0.01 µM of forskolin.
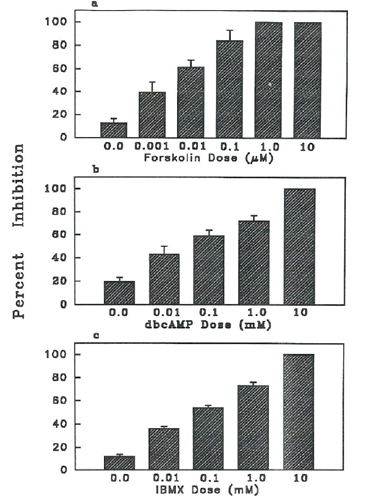
Figure 1 Effects of different concentrations of chemical agents that elevate oocyte cAMP with 1 µg/ml MIS (17, 20 P) stimulation of maturation in denuded goldfish oocytes. Oocytes cultured at zero doses were MIS stimulated and served as control. Results show that elevating cAMP inhibited maturation. a) Percent inhibition by forskolin, activator of adenylate cyclase. b) Percent inhibition on maturation by dbcAMP, an analog of cAMP. c) Percent inhibition on maturation by IBMX, a cAMP phosphodiesterase inhibitor.
As shown in Figure 2, spontaneous maturation (control) of denuded goldfish oocytes was almost 60%. A concentration of 0.01 µM forskolin significantly decreased (P<0.05) spontaneous maturation of denuded oocytes to 20% (Figure 2). Forskolin also significantly decreased (P<0.05) MIS-stimulated maturation from almost 100% to that of the control level (Figure 2).
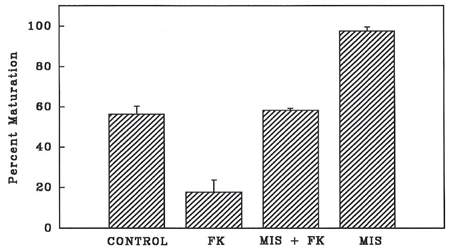
Figure 2 Percent maturation of denuded oocytes stimulated with MIS (17, 20 P) at 1 µg/ml only, MIS in the presence of forskolin (FK) at 0.01 µM, and in 0.01µM forskolin only. GVBD was scored after 24 h. Oocytes cultured without any stimulation served as control.
Figure 3 shows the effects of 10 µM forskolin (FK) added in separate cultures of MIS-stimulated denuded goldfish oocytes at 4 h intervals, and after which, incubated further for 24h.
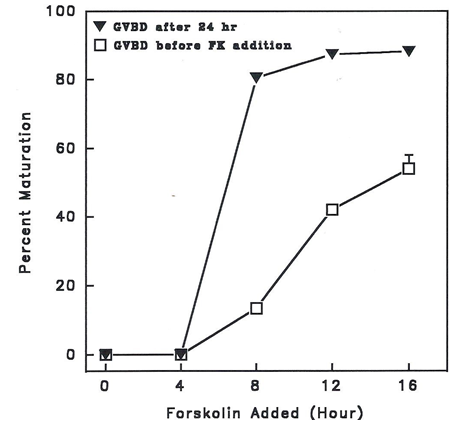
Figure 3 Effect of 10 µM forskolin (FK) on percent maturation of MIS (17, 20 P at 1 µg/ml) - stimulated denuded goldfish oocytes. FK was added at 4 h interval in separate cultures, after which were incubated further for 24 h. GVBD was scored before FK addition and after 24 h. FK completely inhibited maturation up to 4 h post MIS stimulation.
Forskolin added up to 4h after MIS stimulation inhibited maturation completely. At these time periods also, there was no oocyte maturation as indicated by GVBD assay before FK addition. GVBD was observed only after 4h in control incubates before FK addition. Addition of forskolin at these time periods when GVBD was observable did not inhibit MIS- induced maturation. The percentage maturation after 24h incubation even with added forskolin from 8 h post MIS stimulation was significantly different (P<0.05) from that before forskolin addition.
Effect of agent that lower oocyte cAMP
The chemical used was an inhibitor of adenylate cyclase, 2’- deoxyadenosine – 3’- monophosphate. Percent maturation increased with increasing concentrations of the inhibitor (Figure 4). A concentration of 0.1 mm enhanced spontaneous maturation of goldfish oocytes significantly (P<0.05). The percent maturation induced by the inhibitor was comparable to that of MIS-induced maturation.

Figure 4 Effect of 10 µM forskolin (FK) on percent maturation of MIS (17, 20 P at 1 µg/ml) - stimulated denuded goldfish oocytes. FK was added at 4 h interval in separate cultures, after which were incubated further for 24 h. GVBD was scored before FK addition and after 24 h. FK completely inhibited maturation up to 4 h post MIS stimulation.
Cyclic AMP assay
The cAMP levels in goldfish oocytes and follicular layers cultured in vitro are shown in Figure 5. In denuded oocytes cultured with or without free follicular layers, the cAMP content decreased significantly (P<0.05) within 30 min and remained low until the 20h observation period. In contrast, intact oocytes showed a fluctuating pattern of cAMP levels (Figure 5a). A significant decrease (P<0.05) was noted at 4h, after which cAMP increased to basal level (0 h level) at 8h, and decreased again significantly (P<0.05) at 20h. The total cAMP concentrations in the intact oocytes were significantly higher (<P0.05) than those of the denuded oocytes whether these were cultured with or without free follicular layers. The follicular cAMP was about 5 to 10 fold higher than those of oocytes (Figure 5a). However, the presence of follicular layers did not increase the cAMP content of co-cultured denuded oocytes.
In the medium, cyclic AMP concentration was very minimal, except at 0h (Figure 5b). Cyclic AMP levels progressively decreased until 4h and then remained low until the 20h observation period. There were no apparent differences in the overall levels of cAMP in the media of the different kinds of oocytes.

Figure 5 Cyclic AMP levels (p mole/mg protein) assayed in goldfish oocytes.
a) Cyclic AMP levels in oocytes and follicular layers cultured in vitro without MIS stimulation.
b) Cyclic AMP levels in media of oocytes cultured in vitro without MIS stimulation.
c) Cyclic AMP levels in denuded oocytes stimulated with MIS (1 µg/ml) only, MIS with 0.01 µM forskolin (FK), and 0.01 µM FK only.
Intraoocyte cAMP was also assayed in denuded goldfish oocytes stimulated with MIS only, MIS in the presence of forskolin, and forskolin only (Figure 5c). There was an immediate significant decrease (P<0.05) in intraoocyte cAMP within 15 min in MIS-stimulated (1 µg/ml) denuded oocytes. It continued to decrease until 4h, by about 73% from the basal level (at 0h), and began to rise again back to the basal level at 16h. In MIS (1 µg/ml 17,20 P) and forskolin (0.01 µM)-stimulated denuded oocytes, the decrease in intraoocyte cAMP became significantly lower (P<0.05) than the basal level at 1h. Intraoocyte cAMP decreased greatly at 4h, then it increased again but way below the basal level at 16h. In forskolin-treated denuded oocytes, changes in intraoocyte cAMP were not significant (P>0.05). The decrease in intraoocyte cAMP within 15 min was only 30% from the basal level as compared to 52% in MIS-stimulated denuded oocytes. The decrease in intraoocyte cAMP in denuded oocytes treated with MIS and 0.01 µM forskolin was 25% within 15 min, by 41% at 1 h, and 65% at 4h. In denuded oocytes that underwent spontaneous maturation (without MIS), intraoocyte cAMP decreased only by 27% from the basal level in 15 min but subsequently decreased to a more significant low level until the end of the experiment.
The results presented here show that chemical agents able to elevate the intraoocyte cAMP levels such as dbcAMP, IBMX and forskolin, block MIS-induced maturation in denuded goldfish oocytes in vitro (Figure 1). This is consistent with the findings that elevated levels of cAMP are inhibitory to maturation.18,19
Derivatives of cAMP such as dbcAMP have an inhibitory effect on oocyte maturation at concentrations greater than 10-3 M.9 As observed also in this study, a 10 mm dbcAMP would completely inhibit MIS-induced maturation of denuded goldfish oocytes. The inhibition of maturation caused by dbcAMP could be due to its direct action on the oocyte, maintaining it in meiotic arrest.20
The site of action of phosphodiesterase (PDE) inhibitor, IBMX is the oocyte PDE.21 A concentration of 10 mm IBMX completely inhibits MIS-induced maturation of goldfish oocytes. This concentration is rather high if compared to the 0.3 mm concentration of IBMX, which inhibits 75% of basal activity of oocyte PDE in Xenopus.21 In partially denuded trout oocytes stimulated with MIS (17, 20 P at 25 ng/ml) complete inhibition was observed at 0.1 mm IBMX.22 Addition of IBMX inhibits oocyte PDE, resulting to increased level of intraoocyte cAMP and signaled the oocyte to be still in meiotic arrest.
Forskolin, an activator of adenylate cyclase is effective in inhibiting MIS-induced maturation, as well as spontaneous maturation in denuded goldfish oocytes (Figure 1a and 2). It can inhibit maturation completely given at 10 µM up to 4h after MIS stimulation (Figure 3). If a relative time scale is applied in which the period from hormone addition to 50% germinal vesicle breakdown (GVBD50) is normalized to a scale of 0-1.0,23 forskolin is completely inhibitory when added during the first 28% of in vitro meiotic maturation (GVBD50 at 14h). In partially denuded brook trout oocytes stimulated with MIS (17, 20 P) at 5 ng/ml, 10 µM forskolin is completely inhibitory when added during the first 33% of maturation or up to 12 h post steroid stimulation.22 In denuded Xenopus oocytes stimulated with 1.0 µM progesterone and added hourly with 75 µM forskolin, inhibition of maturation was 80% or greater if added during the first 38% of meiotic maturation.24 In Rana pipiens, forskolin is an effective inhibitor when added up to 2 h after progesterone treatment.25
The changes occurring during this early event of maturation can still be overcome by forskolin. After such time, the oocyte becomes irreversibly committed to resume meiosis. Even elevating the cAMP level will not reverse this commitment. In this study, forskolin inhibits goldfish oocytes from resuming meiosis (Figures 2 & 3) until 4h, the time the oocytes become irreversibly committed to proceed to maturation.
The decline in intraoocyte cAMP levels can be causally related to the commitment period.26 In goldfish oocytes, the greatest decrease in cAMP in both MIS and MIS plus FK treated denuded oocytes occurs at 4h, after which cAMP level rises again back to basal level as shown in Figure 5c. Hence, the commitment period in goldfish oocytes occurs when there is a significant drop in cAMP, which is at 4h. After the 4h, the oocytes become committed to resume meiosis that addition of forskolin can no longer inhibit MIS-induced maturation.
It is shown that following MIS stimulation the level of intraoocyte cyclic AMP decreased (Figure 5) and this could be via the inhibition of adenylate cyclase activity.27,28 To show that the same mechanism might be involved, denuded goldfish oocytes were stimulated with 2’-deoxyadenosine-3’-monophosphate, a P site agonist and a natural inhibitor of adenylate cyclase.29 The P site is the intracellular binding site associated with the catalytic unit of adenylate cyclase. The P site agonist, 2’-deoxyadenosine- 3’-monophosphate, has enhanced spontaneous maturation of denuded goldfish oocytes (Figure 4). The percent maturation induced by a concentration of 0.1mm of the agonist is comparable to that induced by MIS at 1 µg/ml. This is evidence that the mechanism of inhibition of adenylate cyclase by MIS and P site agonists are similar. This also confirms the findings by Sadler and Maller27 that the mechanism by which an MIS acts on adenylate cyclase is in common with the P site inhibition.
Stimulation with MIS results in a greater decrease in intraoocyte cAMP that drives the early commitment of MIS-stimulated denuded goldfish oocytes to resume meiosis. As shown in Figure 5c, intraoocyte cAMP did decrease significantly by 52% within 15 min after MIS addition while without MIS, cAMP decrease of about 43% occurred within 30 min. Addition of 0.01 µM forskolin to MIS further delays the onset of cAMP decrease to a significant low level by 1h. This confirms then, that MIS inhibits adenylate cyclase activity, which results in a more rapid decrease of intraoocyte cAMP as compared to just as a response to removal of follicular wall (Figure 5a).
Denudation of oocytes also causes an immediate decrease in the intraoocyte cAMP but only by 27% as compared to 52% in MIS-stimulated denuded oocytes (Figure 5a). In unstimulated intact goldfish oocytes, the cAMP content is higher than that in denuded oocytes which means that a portion of the intraoocyte cAMP may come from the adjacent follicular cells. The cAMP generated by the follicular cells is passed on into the oocyte, not extruded into the medium for the cAMP levels in the media of both intact and denuded oocytes are relatively low (Figure 5b). The low level of intraoocyte cAMP in denuded oocytes is the result of the functional disengagement between the follicular cells and the oocyte. The transfer of cAMP from the follicular cells to the oocyte is disrupted resulting from the removal of the follicular wall. This sharp drop of intraoocyte cAMP in denuded oocytes is responsible for the resumption of meiotic maturation.
The present study confirms the hypothesis that elevated levels of cAMP maintain meiotic arrest. The follicular wall surrounding the oocyte is necessary in the maintenance of meiotic arrest. The follicle cells raise intraoocyte cAMP through gap junction-mediated transmission of follicle cell cAMP to the oocyte.30,31
Many studies have reported a 10-50 percent drop in cAMP levels during maturation. Cicirelli and Smith32 have recorded a 20% decrease in the cAMP content of Xenopus during the first 2-50 min following progesterone addition. Jalabert and Finet33 have also observed a significant decrease in cAMP levels in rainbow trout incubated with 3 µM 17.20 P. Thibier et al.34 have calculated that 10-20% decrease in cAMP levels would be sufficient to trigger oocyte maturation. This explains the spontaneous maturation of denuded goldfish oocytes which shows an immediate decrease in cAMP level. Some (20%) denuded goldfish oocytes treated with 0.01 µM forskolin also undergo maturation (Figure 2). The cAMP concentrations in these forskolin treated oocytes show a decrease of 30% at 15 min and 39% at 4h of incubation (Figure 5c). A low concentration of forskolin, less than 1 µM is effective in inducing maturation.35 The final level of intracellular cAMP elicited by forskolin is determined by the rate of synthesis by adenylate cyclase and the rate of hydrolysis by cyclic neucleotide phosphodiesterases. Such steady-state levels of cAMP are attained after 5 or 10 min in the presence of forskolin. After reaching a maximum, cAMP levels will begin to decline due to cAMP-dependent desensitization of adenylate cyclase or induction of phosphodiesterase activity.36 All these findings confirm the hypothesis that a decrease in cAMP could trigger resumption of meiotic maturation.
The study has shown that a decrease in intraoocyte cAMP is necessary and sufficient to trigger resumption of meiosis that lead to the completion of final oocyte maturation. It demonstrates the possible role cAMP may play in the regulation of oocyte maturation in fish.
Thanks to Prof. T. J. Lam and Prof. C. H. Tan of the Department of Zoology, National University of Singapore, Singapore in whose laboratories I worked out this study.
The author declares there are no conflicts of interest.

©2023 Seraspe. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.