Journal of
eISSN: 2378-3184


Research Article Volume 10 Issue 5
1School of Agricultural Science, Walailak University, Thailand
2School of Sciences, Walailak University, Thailand
3Department of Marine Science, Rajamangala University of Technology, Thailand
Correspondence: Patchara Pedpradab, Associate Professor, Department of Marine Science, Faculty of Sciences and Fishery Technology, Rajamangala University of Technology, Thailand
Received: October 13, 2021 | Published: November 30, 2021
Citation: Yokseng K, Darumas U, Pedpradab P. Culture of a blue marine sponge, Xestospongia sp., in semi-circulate close system stressed with calcium and magnesium concentration. J Aquac Mar Biol. 2021;10(5):230-235. DOI: 10.15406/jamb.2021.10.00326
The goal of this research was to stimulate renieramycin M (RM) production and the growth performance of a blue marine sponge, Xestospongia sp., in response to different concentration of calcium/magnesium (Ca/Mg).The sponge’s pieceswere exposed to various concentrations of Ca/Mg in natural seawater at salinity 35ppt. Results showed that a Ca/Mg concentration at 430/1,230 ppm (T3)stimulated in maximum RM accumulation in sponge tissue (1.74mg/1,500mg tissue), while 410/1,170 (T1),420/1,200 (T2), and a pure seawater control were 0.33, 0.92, and 0.32 mg/1,500mg tissue, respectively. The mean values calculation revealed that there was statistical difference of RM accumulation between T3and control at 95% confidence interval.Sponges supplemented with Ca/Mg at a level of 410/1,170 showed the most growth (3.77g), while 420/1,200, 430/1,230, and the control were 2.36, 2.44, and 1.70g, respectively. The analysis revealed statistically significant growth differentials between T1 and control at the 95% confidence interval. The resultssuggested the Ca/Mg levels are stressor activate secondary metabolites synthesisand promote the growth of a blue marine sponge, Xestospongia sp.
Keywords blue marine sponge, Xestospongia , calcium and magnesium, growth, renieramycins, culture
RM, renieramycin M; DSi, dissolved silicate; DCa, dissolved calcium; DMg, dissolved magnesium
Sponge aquaculture is one of the most effective methods for producing biologically active secondary metabolites with a high potential for use as commercial drug agents. However, this method’s success depends on identifying and understanding the factors that stimulate the production of those metabolites, including environmental stress.1 Stress responses in marine invertebrates induce a physical adaptation at a molecular level,resulting in the synthesis of secondary metabolites that have specific functions.2-5An example of astress-induced defense in terrestrial plants is the de novo biosynthesis of phytoalexins in response to invading fungi).6Another example is the unusual sesquiterpene metabolite, anhuienol, produced by Chloranthus anhuiens is under the stress of heavy metal stimuli.7
In the marine environment, sponges, including their associated microorganisms, produce secondary metabolites through various biosynthetic pathways that are activated by self-defence or stress-induced defence processes; however, this process varies among species.1,8 These metabolites typically have specific ecological roles and other biological functions that are relevant to pharmaceutical applications.Despite numerous secondary metabolites from marine sponges being isolated and characterized unfortunately,only a few studies have described the relationship between their accumulation in tissue and stress or defense for example, defense-driven secondary metabolite synthesis in Aplysinasponges.9-12 Furthermore, the stress-induced secondary metabolites accumulations in marine sponges areless recognizedand remainspoorlyunderstood.In our surveyed for marine sponge in the Andaman Sea, Thailand, we discovered a blue marine sponge, Xestospongia sp.(Figure 1) distributed in the area of Sarai Island, Satun Province (Figure 2).
This blue sponge accumulates secondary metabolites namely renieramycins in their tissue. Theseare marine natural products with chemical and biological activity related to several anticancer drugs, including saframycin, naphthyridomycin, quinocarcin, and ecteinascidin 743. Renieramycins, particularly RM, showed high potency as an antitumor agent against several cancers in concentrations at then anomolar level.13-16Generally, compounds in this group are unstable due to the presence ofan amino alcohol functional group in the molecule. However,it can be stabilized by converting the amino alcohol to an a-amino nitrile by pretreatment with a potassium cyanide (KCN)solution before extraction.17-19Our ultimate goal is the mass production ofRM via culturing a blue sponge in semi-circulate close systemto supply pharmaceutical industriesand laboratories. Therefore, we attemptedto examinethe ecological bases as the primary preparation.We sought to clarify our understandingofthe ionic stress on a blue sponge growth and on RM synthesis.We considered that the stimulation of RM synthesis and growth of blue sponge can be performed by stress induction by changing ionic concentration in seawater particularly calcium and magnesium. These ions present in a regular proportion in seawater. Calcium and magnesium ratios affect the growth and survival of many calcareous marine invertebrates such as corals and foraminifera.20,21For sponges, however, only limited data have been revealed.The functions of calcium ion in marine spongesare regulatingcontraction of the oscular chamber,osmoregulation processand it isan internal mediator of cell aggregation process .22,23While there is less evidence of magnesium’s impact on biological functions, it is an essential ion in osmoregulation and nucleic acid synthesis metabolism .24,25In this paper, wereport the culturing a blue sponge in semi-circulate close system that stress them by using various Ca/Mgconcentrations to observegrowth performance and the accumulation ofRM in tissue.
Sponge preparation
A blue marine sponge, Xestospongia sp., was collected by scuba divers at a depth of 5 m from Sarai Island, Satun Province, Thailand (6°42'51.67" N; 99°50'35.65" E)(Figure 2). Sponge specimens were kept in custom-made boxes during transfer to a marine aquarium and then rehabilitated in a pond (150m3) for 10 days. After an acclimation period, the sponges were considered to be healthy by observing their shape and the presence of a brilliant blue color. Before use, the presence of RM in tissue was analyzed by liquid chromatography-mass spectrometry (LCMS).
Experimental design
A randomized complete block design (RCBD) was used for the experimental design. Three Ca/Mg concentrations, including 410/1,170 (T1), 420/1,200 (T2), and 430/1,230ppm (T3), were prepared as treatments according to the guidelines on elemental requirements of marine organisms.26-28Pure seawater was used as control. Each treatment and control consisted of three replicates.
Culture method
We employeda vertical long-line in a semi-circulate closed system (Figure 3) for the sponge culture. Small pieces of sponge (12g) were cut outof the mother plants and attached to a plastic net with copper wire that was future combined as a rope (one rope containedthree pieces of sponges). The sponge specimens were then placed in a40×60×60cm glass box containing 120,000cm3 of seawater,3replications for a treatmentand control (12 containers totally).Threeropes were cultured in each tank.Magnesium chloride (MgCl2) and calcium chloride (CaCl2) powders were used as sources of calcium and magnesium.Both were measured at the desired concentration before mixing and dissolving in seawater. The waterin the culture tanks was changed to 30% of total volume once per week to remove detritus and residual food particles and replace water lostthrough evaporation.Thelaboratory temperature was maintained at 27±1°C during the experiment period.
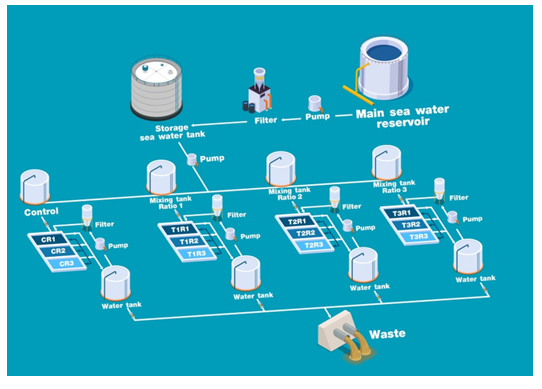
Figure 3 Diagram of semi-circulate close system.Mixing tank ratios 1, 2 and 3 are the Ca/Mg ratios at 410/1,170; 420/1,200 and 430/1,230, were mixed with sea water in each tank, respectively. The letters C, T and R represent control, treatment and replication, respectively.
Growth measurements
The growth of sponges was measured once a week. Samples were weighed and compared with the standard wet-dry weight curve. The wet-dry curve was determined by cutting the sponges into segments of different weights: 2, 4, 6, 8, 10, 12, 14, 16, 18, and 20g. The sponges were then weighed and dried at 80°C for 24hs before weighing. The relationship between the wet and dry weights was then recorded and a linear regression was calculated.
Analysis of RM concentrations
Analysis of the RMwas conducted following the method described by Suwanborirux et al.,17A specimen was cut into fine pieces and accurately weighed to the nearest 150mg. The sample was then macerated with10 mM potassium cyanide in a phosphate buffer solution (6.00ml, pH 7.0) for 5h before extraction with 24ml of methanol. After centrifugation at 5,000rpm for 5min, the supernatant (3.00ml) was partitioned with ethyl acetate (9.00ml). The ethyl acetate layer (3.00ml) was evaporated ina vacuum until dry, andthe dried residue was dissolved in methanol (1.00ml) containing 300mg acenaphthene as an internal standard. The sample solutions were filtered through 0.45nm nylon syringe tip filters before being undergoingHigh-Performance Liquid Chromatography (HPLC; Dionex UltiMate 3000, Thermo Scientific, Waltham, MA, USA). The separation column was achieved using an Acclaim120, C18 reversed-phase column (5µm, 4.6x150mm, Thermo Scientific, Waltham, MA, USA)eluted witha mixture ofmethanol and water (7:3,v/v) at a flow rate of 0.70ml/min.A photodiode array detector was set at a wavelength of 270nm.The RMat a concentration of 1mg/ml was used as a standard.The presence of RMwas confirmed in all samplesby subjecting them to LCMS. LCmeasurementswere carried out using a1290Infinity (Agilent Technologies, Santa Clara, CA, USA).AZORBAX SB-C18 was used as a separation column (Rapid ResolutionHD, 2.1x150mm, 1.8µm; Agilent Technologies, Santa Clara, CA, USA) with aninjection volumeof 10µl. The LC system was connected to the Agilent Technologies 6490 Triple Quadelectrospray ionization mass spectrometer (ESIMS; Agilent Technologies, Santa Clara, CA, USA). Both negative and positive modes were detected.
Water qualitymeasurement
Physico-chemical parameters, including salinity, temperature, dissolved oxygen, and pH, were determined using an autoanalyzer (Global Water Instrumentation, Phoenix, AZ, USA). Dissolved silica, calcium, magnesium, ammonia, nitrite, nitrate, and phosphate in culture water were analyzed weeklyfollowing the method described by American Public Health Association.29
Statistical analysis
The statistical analysis was conducted usingSPSS 20.0 software (IBM Corp., Armonk, NY, USA). Analysis of variance (ANOVA) and Tukey’stest was used to compare the mean values.
The existence of RM in the sponge mother plant:The target sponge colony was examined for the presence of RM using HPLC and LCMS. The LCMS of a standard RM showed [M+H]+ at m/z576.2399 compatible with molecular C31H33N3O8. The HPLC chromatogram showed a peak at retention time (Rt) of 3.097min (Figure 4).

Figure 4 HPLC chromatograms (A and B) and mass spectra data (C and D) of standard RM and sponge’s mother plant. A and C were a standard RM; B and D were the extracts of sponge’s mother plant.
RM synthesis and accumulation in sponge tissue
The results showed that sponges stressed with Ca/Mg at 430/1,230 ppm (T3) gave maximum RM accumulation at 1.74 mg/1,500 mg tissue, while 410/1,170 (T1), 420/1,200 (T2), and control were at 0.33, 0.92, and 0.32 mg tissue, respectively. The mean values calculation revealed that there were statistical difference of RM accumulation between T3 and control at 95% confidence interval, but do not show the different among treatments (T1,T2, and T3) (Figure 5).
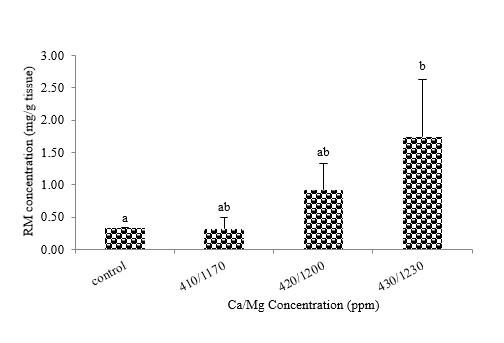
Figure 5 RM concentrations in a sponge’s tissue.Mean values (±SE) represent by bars. The difference letters mean statistically significant at 95% confident interval (p<0.05).
Growth
The relationship between the wet and dried weights was measured and plotted, as shown in Figure 6. The sponges stressed with Ca/Mg concentrations at a level of 410/1,170 showed the maximum growth (3.77g), while 430/1,230, 420/1,200, and control were 2.44, 2.36, and 1.70g, respectively. Statistical analysis revealed the growth was significantly different between groups that stress with Ca/Mg 410/1,170 and control at a 95% confidence interval (Figure 7), but it does not show the difference among treatment groups.
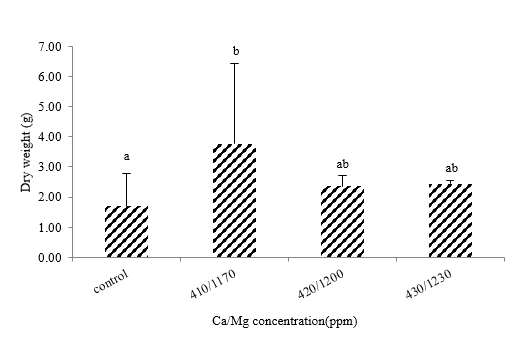
Figure 7 Growth of cultured sponges.Mean values (±SE)represent by bars. The difference letters mean statistically significant at 95% confident interval (p<0.05).
Water quality
Measured water quality was not statistically different at the 95% confidence interval.The exceptions were light intensity, dissolved silicate, calcium, and dissolved magnesium, whichshowed statistical differences at the 95% confidence interval (Table 1).The mean value of light intensity in all experiment units was 993.81±0.17 lux. There wasno differencebetween the control group and the third treatment (Ca/Mg, 430/1,230).However,both of those valuesdifferedfrom the firsttreatment (Ca/Mg at 410/1,170). Throughout the experiment period, the dissolved silicate concentration variedby 0.48±0.08mg/L, whilethe control group and the first treatment (Ca/Mg at 410/1,170)differed.Calcium concentrationsin all treatments showed statistical differences.Onlythe T3 showed a difference in control values.For dissolved magnesium, the values were divided into two different groupsthe first being 1,175.72±6.55 and 1,197.14±2.48, and the second being 1,132.86 ± 9.90 and1,152.86±11.33.99.
The presence of the mother plant sponge
The HPLC chromatogram of a standard RM showed the Rt peak at 3.097min(Figure 4). However, the mother colony showed the Rt peak at 2.847min (Figure 3. B),representing a signal delay of 0.250 min because of impurity noise.Furthermore, the present of RM was confirmed by LCMS/MSspectral data that revealed the m/z 576.2343.
RM accumulation in a blue sponge’s tissue
The synthesis of secondary metabolites in marine sponges is generally stimulated by defense and environmental stress processes.10,12 Due to the body structure of sponges, they are the habitat of many associated microorganisms, thus some secondary metabolites are produced by these associated microorganisms. Renieramycins contained in the tissue of a blue Xestospongia sponge were also found in another sponge. It was found in a Haliclona sponge and was reported to be produced by a non-culturable symbiotic microorganism, CandidatusEndo haliclona reniera mycinifaciens,30 suggesting that renieramycins in a blue Xestospongia sponge might be produced by a similar associated microorganism. However, ionic stress can occur in both the host and associated microorganisms.In our case, RM accumulation in a blue Xestospongia sponge was associated with ionic stress. Calcium and magnesium were added at higher concentrations than that of the ambient environment; hence the ionic balance of the sponge and its associated microorganisms was interrupted, resulting in a stressful condition. To maintain the ionic balance, the secondary metabolite was produced and accumulated in their cells.31This revealed by high RM accumulation in the sponge tissue when stimulated with a higher Ca/Mg concentration as illustrated in Figure 5.
Growth performance
On a cellular level, the sponge’s growth is regulated by certain metabolic factors through cell division, membrane transport, enzyme activity, and cell aggregation, all of which involve divalent ionic molecules such as calcium or magnesium ions.32 In stimulus-response cell aggregation,calcium ions play an essential role as intracellular messengers, while magnesium ions are primarily involved in membrane transport.22 Calcium ions appear to function more in sponge metabolism than magnesium.33 Based on our measurements, the highest growth occurred when supplementing with a Ca/Mg concentration of 410/1,170ppm. At other levels (370/1,170; 420/1,200 and 130/1,230), the increase did not result in different values (Figure 7),however there was no statistical difference among the group of Ca/Mg supplementation, but differ to control group (no calcium and magnesium were added). This variance indicated that the blue Xestospongia sponge’s growth needs to supplement with calcium and magnesium on some optimum ranges depend upon the ionic starvation in cultured water. Based on the results, Ca/Mg was vital to the growth of marine sponges. This was the case for Ephydatiafluviatilis, which was cultured in laboratory conditions under varying concentrations of calcium and salinity. The results suggested that the sponge’s growth rate increased relative to calcium concentration, but the growth rate decreased with increasing salinity. In our experimental system, salinity was controlled at a constant value 32.64-34.67mg/L (Table 1). Other toxic substances in water such ammonia and nitrite were under control. Therefore, those factors would not have affected the growth of the blue sponge, excepting Ca/Mg concentration. The contraction of the oscula membrane in marine sponges stopped when either calcium or magnesium was omitted.31 These contractions involve water movement and feed transportation, further captured by choanocyte cells in the mesohyl layer. Therefore, higher calcium ion concentrations should increase feed capturing. However, this experiment found that excessively high Ca/Mg concentration resulted in decreased sponge growth (T3), because the oscula membrane was unbalanced and interrupted.25In addition, magnesium is the most abundant intracellular divalent ion and plays a central role in many cellular processes. Magnesium has been implicated in the activation of a large number of enzymes, hormonal signaling features, protein synthesis, and cell division34 These effects may relate to the growth of marine invertebrates, such the blue sponge.Comparing the relative growth to RM synthesis (Figure 8), high levels of RM was recorded in sponge’s tissue when exposed to higherCa/Mg concentrations not for growth performance. This data support that the capacity of secondary metabolites accumulation in this blue sponge depending on stress of the sponge and/or theirassociated bacteria.
Water quality
Water qualityvalues in each experimental unit were in the standard seawater range (Table 1) and not statistically different, with the exception offour parameters: light intensity, dissolved silicate, calcium, and dissolve magnesium levels. For light intensity, we installed 195-watt light-emitting diodes (LED)on the laboratory ceiling as a light source.Each experimentalunit (culture container) was exposed to slightly unequal light intensity depending on their position. However, we controlled the light intensity in the range of 993-997 lux according to the requirements of the blue Xestospongia sponge. Light intensity affects the survival and death of sponges.The associated microorganisms in phototrophic sponges capture light energy to synthesize organic molecules that will be further taken up by the host.Less-than-optimum light intensity (<0.8mol photons m−2d−1) affectedtheassociated microorganismsas found in the sponges Clionaorientalis and Carteriospongia foliascens; they became discolored (bleached) over 28 days(Pineda et al., 2016).35 In the bluesponge,we observed bleaching(Figure 8) when exposed to a light intensity of<900 lux at a temperature <27°C; normally, optimum temperature for growth and RM accumulation in a blue Xestospongis sp. was in the range of 26-29°C.35 The mean values of dissolved silicate in the control group differedfrom the T1 (Ca/Mg, 410/1,107).The most silicate was uptakeby the sponge in the T1, while uptake for the control group wasthe least.This variation implied thatthe ionic stress stimulated the rate of silicate uptake in the marine sponge Xestospongia sp.
In the marine environment, the demand for dissolved silicate (DSi) by sponges varies by species, each with a different DSi saturation point.AhighDSilevel stimulates the sponge, increasing the assimilation rate found in Haliclonasimulans, which had a saturation point of about 70mM.36 On the other hand, DSi starvation in seawater caused the assimilation rate to decrease.37 Therefore long-term cultivation must supply silica in the cultural water. In our experiment, we found that a blue sponge assimilated low levels ofDSi in ambient conditions (control), but higher levels when stressed with Ca/Mg (T1).However, the concentration of Ca/Mg is too high causing the absorption of DSi to be low as shown in treatments 2 and 3 (Table 1). The pattern of dissolved calcium (DCa) was similar to that of dissolved magnesium (DMg).TheDCa values of T1 were lower thanthe controlgroup and other treatments.Absent a report describing the physiology of calcium uptake by marine sponges, we assume that this phenomenon may relate to the stress effect and overdose of Ca/Mg. High Ca concentrationsin the cultured environment (T2 and T3) stressed the sponge,resulting in low assimilation.In contrast,Ca contained in T1 should have been at the optimum levelto stimulate the assimilation of a blue sponge because the concentration in cultured water remained low.Like Calcium, stress also affectedthe assimilation of magnesium (Mg).As revealed in T3, the capacity for uptake was stimulated at low Mg levelsbut decreased when exposedto high Mg concentrations.38,39
Water quality parameters |
Control |
Ca/Mg |
p-Value |
||
410/1170 (T1) |
420/1200 (T2) |
430/1230 (T3) |
|||
Salinity(ppt) |
33.87 ± 0.96a |
33.94 ± 0.03a |
33.87 ± 0.08a |
33.82 ± 0.02a |
NS |
Temperature(°C) |
28.33 ± 0.18a |
28.42 ± 0.29a |
28.21 ± 0.21a |
28.20 ± 0.49a |
NS |
pH |
8.34 ± 0.01a |
8.31 ± 0.03a |
8.32 ± 0.03a |
8.33 ± 0.03a |
NS |
Light(lux) |
996.09 ± 0.83b |
993.81 ± 0.17a |
995.14 ± 1.43ab |
996.67± 0.17b |
* |
Ammonia(mg/L) |
0.31 ± 0.04a |
0.31 ± 0.04 a |
0.29 ± 0.00 a |
0.29 ± 0.00a |
NS |
Nitrite(mg/L) |
0.02 ± 0.01a |
0.03 ± 0.02a |
0.05 ± 0.03a |
0.02 ± 0.01a |
NS |
Nitrate(mg/L) |
3.57 ± 1.79a |
6.35 ± 1.71a |
4.76 ± 1.03a |
4.17 ± 1.03a |
NS |
Phosphate |
0.02 ± 0.01a |
0.01 ± 0.00a |
0.01 ± 0.01a |
0.02 ± 0.01a |
NS |
Silicate(mg/L) |
0.48 ± 0.08b |
0.24 ± 0.09a |
0.34 ± 0.08ab |
0.34 ± 0.08ab |
* |
Carbonate |
6.52 ± 0.08a |
6.48 ± 0.08a |
6.52 ± 0.08a |
6.38 ± 0.08a |
NS |
DO(mg/L) |
7.43 ± 0.29a |
7.96 ± 0.02a |
7.90 ± 0.22a |
7.76 ± 0.21a |
NS |
Calcium(mg/L) |
412.38 ± 2.18bc |
391.11 ± 0.96a |
410.85 ± 0.75b |
415.24 ± 1.64c |
* |
Magnesium(mg/L) |
1175.72 ± 6.55b |
1132.86 ± 9.90a |
1152.86 ± 11.33a |
1197.14 ± 2.48b |
* |
|
|
|
|
|
|
Table 1 Mean values of water quality in culture tanks
Note. NS in the same row are not statistical different (p<0.05), a symbol * is statistical different (p<0.05). Mean value in the same row with different superscripts sucha,b,c are significant different atp<0.05.The acronym “T1-T3” is treatment 1-3, respectively.
This experiment was designed to determine the calcium and magnesium stress on RM synthesis and growth performance of a marine sponge. In the blue Xestospongia sponge, Ca/Mg concentrations are involved in growth and RM synthesis.A high level of RM in sponge’s tissue was observed when supplemented with higherCa/Mg concentrations, this suggested that the sponge or/and associated bacteriawere under stress condition causing to induce the RM synthesis. Growth of sponge was response to an optimum Ca/Mg concentration, while the excessive concentrations caused growth decreasing. Water quality in all experimental units were not significant difference exception of four parameters light intensity, dissolved silicate, calcium, and magnesium levels. This finding concluded that an optimum Ca/Mg concentration was necessary to stimulate a blue Xestospongia sponge growing, while higher level of Ca/Mg concentration induces sponge or/and associated bacteria to produce RM.
None.
None.
The author declares that there are no conflicts of interest.

©2021 Yokseng, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.