Journal of
eISSN: 2378-3184


Research Article Volume 12 Issue 1
Caspian Sea Ecology Research Center (CSERC), Iranian Fisheries Science Research Institute (IFSRI), Agriculture Research, Education and Extension Organization (AREEO), Iran
Correspondence: Seyyed Mohammad Vahid Farabi, Caspian Sea Ecology Research Center (CSERC), Iranian Fisheries Science Research Institute (IFSRI), Agriculture Research, Education and Extension Organization (AREEO), Mazandaran province, Sari, Iran
Received: December 11, 2022 | Published: January 2, 2023
Citation: Farabi SMV, Roohi A, Azari A. Comparative study of the impacts of fish cage culture on some environmental factors and Macrobenthic communities in offshore farms in the southern Caspian Sea. J Aquac Mar Biol. 2023;12(1):1-9. DOI: 10.15406/jamb.2023.12.00350
The aim of this study was to investigate the effects of fish cage culture on Macrobenthic in the southern Caspian Sea. Samples were collected in two offshore farms with four floating polyethylene cages with a production of 60 tons for 5 months in 2015. Sampling was performed of macrobenthos, physicochemical, and sediment in January, March, May, and August at the depths of shade, 50, 100, and 1000 m in three geographical directions (east, west, and south) of the farm. Macrobenthic analysis showed Hypaniola kowalewskii and Streblospio gynobranchiata were predominant, which resulted in an unusual condition due to the effectiveness of the marine farm. There was a linear relationship between environmental factors and macrobenthos communities under the RDA analysis test and the water temperature was the most crucial factor in the density of the dominant species in both fish farm sites. The dominant Macrobenthic species were subjected to environmental conditions in addition to the influence of temperature, salinity, pH, dissolved oxygen, nitrogen, and phosphorus compounds. This situation can be attributed to the hydrological conditions of the studied area; low production of fish, a short period of fish farming, suitable depth of cages established and different water flow directions.
Keywords: fish cage culture, macrobenthic, offshore farm, Caspian Sea
By increasing the human population along with food requirements due to the limitation of freshwater in the world caused human attention to water resources of seas and oceans to provide the required protein.1 Therefore, the rate of aquaculture growth in the world in the last thirty years has been due to cage fish farming in marine environment.2 Freshwaters limited from the past in Iran (with an average rainfall of 240 mm compared to world average rainfall of 850 mm,3 it always is limiting factor for expanding agriculture and industry, even social.4 Therefore, the future of aquaculture development in Iran depends on the marine water and fish cage culture. On the other hand, any sustainable development of aquaculture requires that its environmental effects should be considered effectively, in particularly, according to many experts, a large part of future growth in the world of aquaculture is related to the marine ecosystems.5 According to White,6 the status of environmental impacts in marine fish farms, must pay attention to two important points: basic environmental studies and the range capacity of the area that receives the impacts. Environmental Impact Assessment (EIA) is the only way for aquaculture environmental management and should be adapted to the ecological conditions of the area. EIA legal requirements usually focus on intensive aquaculture, especially shrimp and fish farming in sea cages. Usually, in evaluating environmental goals and quality standards and criteria are considered by studying on benthic organisms and their dispersion.7
Since 1990, large communities of benthic invertebrates were commonly used to assess the ecological status of aquatic ecosystems.8 These organisms are more sensitive than other aquatic organisms (algae) due to their special characteristics such as high species richness, habitat, and different reactions to environmental factors. These features have been considered for cumulative effects in the habitat, susceptibility to physicochemical changes and long-life cycle. Recently, large communities of benthic invertebrates have been used to design ecological predictive tools in American and European systems.9
Nabavi et al.,10 to study the frequency and diversity of pollinators, sampled of four stations: sub-cage up to 400 m off in Ghazaleh estuary (in Persian Gulf).
Their results showed that the macroinvertebrates species diversity from the sub-cage station to the last one (400 m forward) increased from 1.79 to 2.11 ind.m-2 and the dominance from 0.41 to 0.16, which was affected by the presence of species resistant to organic substances from the waste of the cages. Jahani et al.,11 expressed the effects of cage fish farming benthic communities by using the BOPA biological index in the Ghazaleh estuary of the Persian Gulf. They were sampled at a site with 9 fish cages at four stations (below the cage, 50, 150 and 400 m off the cage or control) monthly. They showed that the frequency, biomass, and diversity index in sub-cage station were lower than other stations, especially the control station.
Parafkandeh Haghighi et al.,12 noticed the density and biomass of macrobenthos in the fish cage establishment on the southern coast of the Caspian Sea by determining three stations from the center of the cage and at 50 and 500 m away. The results showed that Streblospio gynobranchiata was one of the most abundant populations of benthic organisms with 93.3%. The lowest individuals of macrobenthos were observed under the cage and the highest at 500 m, but the highest number of biomass was observed at the 50 m.
The results of the study showed the effect of rainbow trout cage culture in Canada by Rebecca et al.,13 that after two months of fish farming period, the abundance and reduction of species richness of benthic invertebrates, under cages compared to the distance of 45 m, was reduced by 8 fulfill. They showed that using PCA test, 76% of the changes in the frequency of benthic invertebrates were related to off the cage with chemical variables changes, which were also related to local changes and with a decreased off more than 15 m.
Plavan et al.,14 showed that the cage culture of rainbow trout in floating cages caused a change in the structure of large communities of benthic invertebrates and increased the abundance and biomass of detritus-eating worms were associated with reduced biodiversity and elimination of benthic clean water index species in Romania.
Jahani et al.,15 expressed the effects of fish farming in cages on macrobenthic communities around the cage up to 400m in the Persian Gulf. The results showed that in the area under the cage, the frequency, biomass, and species diversity of macrobenthos were less than away from the cage.
Haddadi Moghadam et al.,16 also showed by examining the effect of rainbow trout farming on macrobenthic communities around the cage, that the amount of species diversity under the cage was more significant than the distances of 150 and 300 m in the Caspian Sea. The predominant groups were bivalves and polychaetes and had low values of ecological indicators in macrobenthos. This study therefore, aimed to compare the effect of rainbow trout culture in two offshore farms on macrobenthos communities in the southern Caspian Sea.
Study Area: This research was conducted in two offshore farms in Kelarabad (46°44.777ʹN, 51°15.813ʹE) and Abbasabad (46°47.201ʹN, 51°7.836ʹE) regions located in Mazandaran (Iran) in the southern Caspian Sea. There were four cages in each farm at a depth of 30 m depth: in the Kelarabad at 3200 m far from the shore, and in the Abbasabad region at 5500 m from the shore. The cages were stocked with rainbow trout (about 250-200 g). The amount of storage in Kelarabad cages (diameter 16 m) was about 15 tons and in Abbasabad cages (diameter 20 m) with 20 tons (48,000 and 62,000 pieces or 2 kg per cubic meter), respectively. The weight gains from the above farms were 48, 60, and 15 tons with an average wet weight of 700-900 g during the 5 months of fish farming (December to May, respectively).
Research methodology
Water Sampling and macrobenthic: A sampling of biological and abiotic factors was concluded in four stages, start of cage culture, (January), in the middle of the rearing period (March), at the end of the rearing period (May) and about three months after (rest time) the end of the rearing period (August), at the places of 5 m (cage shade), 50 m, 100 m and 1000 m (control station) off in three geographical directions (east, west and south) due to the general flow of water for the possibility of the impact on coastal areas.
Water samples for physicochemical parameters at each station were taken (by mixing three samples replicates from depths of surface, 10 m 20 m) using by Ruttner and macrobenthic organisms were collected by Van veen grab (0.1 m2).
Laboratory Study: The study of biotic and abiotic factors was as follows Table 1 (Table 2).
Parameters |
The method of investigation. |
Physicochemical factors of water |
Respectively: Mercury thermometer, Secchi disk, Russian electrosolymer, pH Meter WTW320, Conductivity Meter Hatch, Winkler method, Fenat method, Bern Schneider and Robinson method, Cadmium reduction column method, Mourning correction method, Valderma method, by computational method, Vetzel and Link method40,42 |
Macrobenthic |
The samples were identified using the Caspian Sea benthic book.41 Conversion coefficient was used to convert wet weight to dry weight46 |
Grain Size |
100 g of precipitate was placed in sodium hexametaphosphate (at a concentration of 6.28 g/L) for 12 hours and then passed through a sieve with 1000, 500, 250, 125 and 63 micron meshes, respectively, on an electric shaker, and after drying in the oven, the remaining precipitate on Each sieve was weighed.43 |
TOM (Total Organic Matter) |
The dried sediment in Avon was placed at 75°C (A), then at 105°C (B) for 24 hours. The furnace was placed at 550 °C (C) and the corresponding weights were determined and calculated using the following formula: T.O.M%=(B-C/A-B)*10043 |
Table 1 Measured parameters and quantitative and qualitative measurement method of biotic and abiotic factors
Parameter |
Description |
Parameter |
Description |
Temperature (ºC) |
T. °C |
Organic nitrogen (microgrom/liter) |
N org. org. μg/l |
Transparency (m) |
Trans. m |
Mineral nitrogen (microgrom/liter) |
N inorg. μg/l |
Turbidity (Permeability Unit) |
Turb. NTU |
Total nitrogen (microgram/liter) |
TN μg/l |
Salinity (g/l) |
Sal. PSU |
Organic phosphorus (microgram/liter) |
P org. μg/l |
Electrical conductivity (millis of siemens per centimeter) |
EC ms/cm |
Mineral phosphorus (microgram/liter) |
P inorg. μg/l |
Dissolved oxygen (mg/l) |
DO mg/l |
Total phosphorus (microgram/liter) |
TP μg/l |
PH |
pH |
Total nitrogen to total phosphorus ratio |
TN:TP |
Nitrite (microgram/liter) |
NO2- μg/l |
Total all ingredients (percent) |
TOM % |
Nitrate (microgram/liter) |
NO3- μg/l |
Macrobenthos density (D in m2) |
In Ben. n/m2 |
Ammonium (microgram/liter) |
NH4+ µg/l |
BiomassMacrobentose (g/m2) |
B Ben g/m2 |
Table 2 Symbols used in text, figures, and tables with units of measurement
Calculation of ecological indicators: Ecological indicators were used to investigate the environmental disturbance around the cage. Therefore, with decreasing index numbers, the amount of turbulence increases. Shannon diversity index, Margalf species richness index, and Simpson dominance index were used to calculate ecological indicators.17
Formula 1 Shannon Index
́H:
Shannon index value, i: number of organisms in species, n: number of species in sample, Pi: ratio of number of species I to total number of species Formula 2 Margalf Index
R: Margalf Index, S: Total number of species, N: Abundant total species
Formula 3 Simpson Index
P: The proportion number of organisms dropped from the species, n: the total number of organisms of a particular species, N: the total number of organisms of all species.
Data analysis
SPSS statistical program (Version.22) was used to analyze the data. Kolmogorov-Smirnov test was used to normalize the distribution of the data.18 One-way ANOVA, PCA and multivariate tests were used to analyze the effect of time and distance from shelves on biotic and abiotic (normal) factors.19 In each test, Duncan's multiple range test at 95% confidence level was used to compare the mean after being significant in F test. To determine the focal correlation of environmental factors and the frequency of biological communities was used under multivariate statistical test using Canoco software (Version.4.5) under RDA (Redundancy Analysis).
Factor analysis of physical and chemical factors of water affected by cage culture
Multivariate analysis of physical and chemical factors of water between Kelarabad and Abbasabad regions with depended variables (distance from the cage, geographical direction, and sampling period) showed that there is a significant difference between the two sites based on the sampling period (P>0.05). Despite the difference between two sites on water turbidity (P <0.05), there was no difference between the two sites in terms of different geographical directions and distance from the cage (P >0.05). Thus, turbidity values were determined to be 4.67±0.21NTU in Kelarabad and 5.56±0.19NTU in Abbasabad. In the main component analysis of physicochemical data of water, parameters of electrical conductivity, temperature, salinity, total nitrogen, pH, and organic phosphorus have the highest variance among the main components at 30.23% of the total 84.77% (Table 3).
Parameter |
No. Co. |
1 |
2 |
3 |
4 |
5 |
Variance |
30.23 |
21.37 |
14.38 |
11.1 |
7.67 |
|
EC(ms/Cm) |
|
0.815 |
-0.422 |
0.018 |
-0.251 |
-0.252 |
TDS(g/l) |
|
0.808 |
-0.410 |
0.024 |
-0.279 |
-0.238 |
Water T. (OC) |
|
0.801 |
-0.322 |
0.057 |
-0.229 |
0.263 |
Sal (PSU) |
|
0.780 |
-0.361 |
0.024 |
-0.297 |
-0.166 |
Ntotal(μg/L) |
|
0.686 |
0.197 |
-0.336 |
0.532 |
0.085 |
pH |
|
-0.619 |
-0.443 |
0.374 |
-0.218 |
-0.166 |
Porg(μg/L) |
|
0.558 |
0.539 |
-0.102 |
-0.321 |
0.120 |
Pinorg(μg/L) |
|
0.308 |
0.797 |
0.022 |
-0.108 |
0.067 |
Turb (NTU) |
|
-0.314 |
0.775 |
0.002 |
0.285 |
-0.279 |
Ptotal(μg/L) |
|
0.514 |
0.765 |
-0.052 |
-0.259 |
0.111 |
NH4(μg/L) |
|
0.161 |
0.604 |
0.496 |
0.370 |
0.233 |
Norg(μg/L) |
|
0.314 |
0.106 |
-0.854 |
0.281 |
0.032 |
Ninorg(μg/L) |
|
0.554 |
0.137 |
0.716 |
0.378 |
0.079 |
NO3(μg/L) |
|
0.540 |
-0.025 |
0.568 |
0.480 |
-0.057 |
DO(mg/l) |
|
0.160 |
-0.410 |
-0.423 |
0.239 |
0.394 |
NO2(μg/L) |
|
0.349 |
-0.263 |
0.388 |
0.588 |
0.097 |
Trans. (m) |
|
-0.277 |
-0.268 |
0.181 |
-0.145 |
0.846 |
Table 3 Matrix and variance of the main components of physicochemical factors of water during the fish cage culture
Grain size and total organic material in fish cage culture
The main percentage of granulation was silt-clay particles (<0.063 mm) and sand (0.063-0.125 mm) around cage culture in Kelarabad and Abbasabad (Figure 3).
There was also a significant difference between the composition of the above-mentioned granulation percentage in the sampling period of 82.37 and 15.03 percent in the Kelarabad region with 91.71 and 6.44 percent in the Abbasabad, respectively (P <0.05).
During the sampling of the effect of fish farming on the percentage of sediment texture (silt clay and sand) in Kelarabad, the highest percentage of silt clay and the lowest percentage of the sand was measured in May (P <0.05), but the was no significant difference from the other sampling periods (P <0.05).
But in Abbasabad, unlike Kelarabad, the lowest percentage of silt clay and the highest percentage of sand was observed in May (P <0.05) with no significant difference in other sampling periods (0.05<P) (Figure 1).
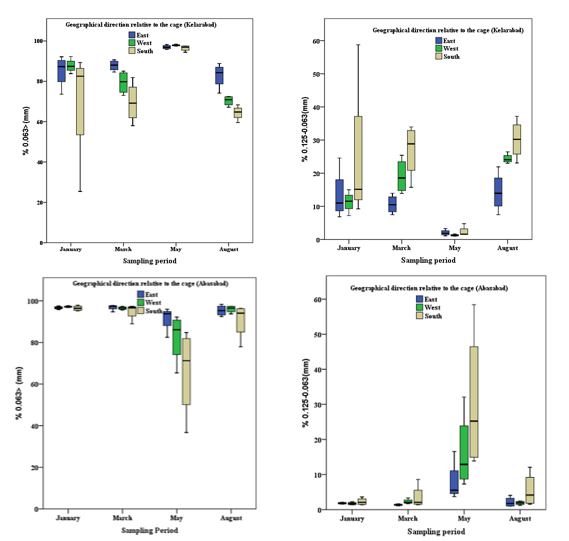
Figure 1 Comparison of aggregation (silt-clay: 0.063> and sand: 0.063-0.125) substrate sediment in different directions during sampling in two cage culture in the southern of Caspian Sea ¬(Average± SE).
The effect of fish cage culture on the accumulation of total organic material at different distances from the cage at each sampling period in the two offshore farms was not significant (P<0.05) (Figure 2). However, there was a significant difference between sampling periods around each farm and between the two farms, by sampling months (P<0.05).
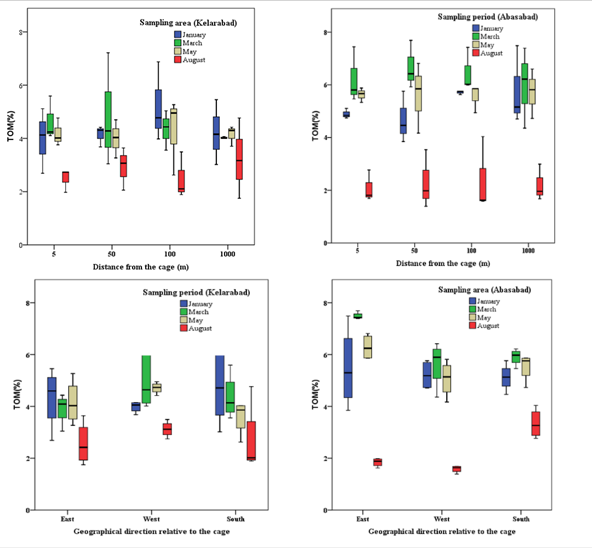
Figure 2 The effect of fish cage culture on inorganic changes in two cage culture in the southern of Caspian Sea (Average± SE).
The effect of fish farming on macrobenthic communities around the cages
In this study, three branches, six categories, eight orders, nine families, 10 genus and species were identified (Table 4).
Branches |
Category |
Order |
Family |
Genus and species |
Annelida |
Polychaeta |
Sponoida |
Sponoidae |
Streblospio gynobranchiata |
Sedentary |
Amphartidae |
Hypaniola kowalewskii |
||
Aciculata |
Nereidae |
Hediste diversicolor |
||
Oligochaeta |
- |
- |
- |
|
Arthropods |
Crustacea |
Cumacea |
Psudocumidae |
Schizorhynchus eudorelloides |
Pseudocuma grasiloiedes |
||||
Cirripedia |
Balanidae |
Balanus improvises |
||
Insecta |
Diptera |
Chironomidae |
Chironomus albides |
|
Amphipoda |
Malacostraca |
Cardiophilus baeri |
||
Niphargoides similis |
||||
Mollusca |
Bivalvia |
Veneroida |
Cardidae |
Cerastoderma glucaum |
Table 4 Macrobenthos around two cage culture in the southern of Caspian Sea
The highest percentage of macrobenthos density around two marine farms was related to Streblospio gynobranchiata with 61.08% and 52.78% and the highest percentage of biomass frequency in Kelarabad was Cerastoderma glucaum with 71.28% and Hypaniola kowalewskii with 40.7% (Figure 3).
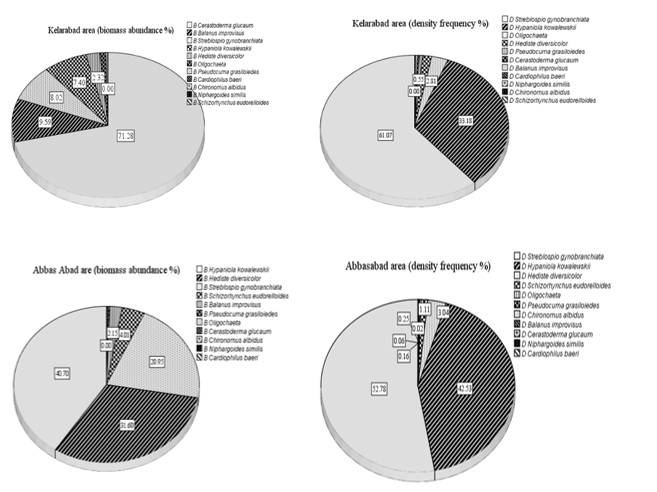
Figure 3 Biomass and abundance of macrobenthos percentages around two cage culture in the southern Caspian Sea.
The density and biomass values of macrobenthos were not significantly different between the two offshore farms during the sampling period (P<0.05) (Figure 4).
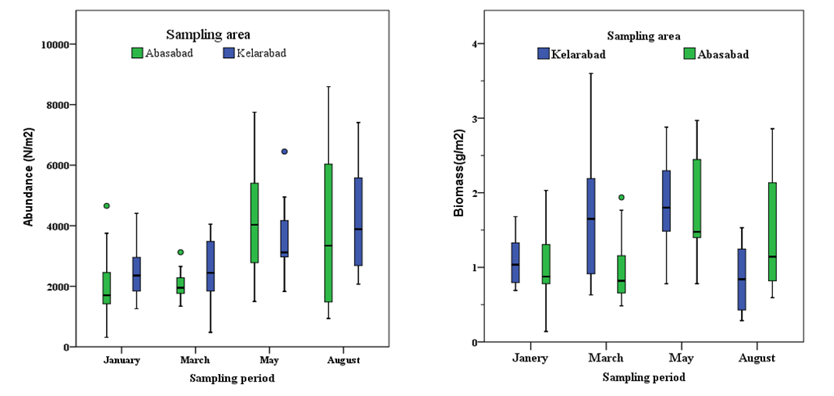
Figure 4 Density and biomass of macrobenthos around two caged fish farms in the southern Caspian Sea (Aaver ± SE).
The effect of cage culture on macrobenthos indices
The effect of cage culture in two offshore farms on biological indicators in the distance from the cage and different geographical directions by sampling months under multivariate test did not show a significant and regular difference (P>0.05) (Table 5).
Index |
|
Distance (m) |
January |
March |
May |
August |
Shannon |
Kelarabad |
5 |
0.63±0.21 |
0.76±0.16 |
0.74±0.01 |
0.46±0.22 |
50 |
0.67±0.18 |
0.96±0.26 |
0.82±0.11 |
0.34±0.10 |
||
100 |
0.72±0.50 |
0.84±0.15 |
0.78±0.05 |
0.29±0.19 |
||
1000 |
0.59±0.31 |
0.85±0.11 |
0.91±0.11 |
0.39±0.28 |
||
|
5 |
0.87±0.04 |
0.70±0.05 |
0.64±0.05 |
0.49±0.27 |
|
50 |
0.93±0.09 |
0.87±0.17 |
0.13±0.08 |
0.35±0.22 |
||
100 |
0.91±0.07 |
0.80±0.11 |
0.87±0.26 |
0.27±0.09 |
||
1000 |
0.90±0.03 |
0.87±0.15 |
0.82±0.16 |
0.46±0.19 |
||
Marbles |
Abbasabad
|
5 |
0.30±0.07 |
0.30±0.20 |
0.36±0.11 |
0.24±0.13 |
50 |
0.33±0.07 |
0.40±0.25 |
0.40±0.07 |
0.32±0.05 |
||
100 |
0.40±0.26 |
0.33±0.07 |
0.35±0.13 |
0.20±0.07 |
||
1000 |
0.30±0.09 |
0.42±0.06 |
0.45±0.08 |
0.34±0.12 |
||
Kelarabad |
5 |
0.72±0.07 |
0.55±0.07 |
0.57±0.14 |
0.51±0.13 |
|
50 |
0.73±0.19 |
0.71±0.24 |
0.69±0.08 |
0.55±0.04 |
||
100 |
0.57±0.07 |
0.64±0.07 |
0.77±0.13 |
0.54±0.11 |
||
1000 |
0.49±0.02 |
0.70±0.04 |
0.78±0.04 |
0.53±0.09 |
||
Simpson |
Abbasabad
|
5 |
0.63±0.13 |
0.53±0.07 |
0.56±0.02 |
0.73±0.27 |
50 |
0.63±0.11 |
0.43±0.08 |
0.52±0.04 |
0.83±0.05 |
||
100 |
0.62±0.24 |
0.47±0.05 |
0.51±0.01 |
0.85±0.11 |
||
1000 |
0.67±0.19 |
0.47±0.05 |
0.50±0.04 |
0.82±0.13 |
||
Kelarabad |
5 |
0.49±0.07 |
0.56±0.06 |
0.62±0.04 |
0.74±0.16 |
|
50 |
0.49±0.06 |
0.49±0.07 |
0.52±0.07 |
0.80±0.14 |
||
100 |
0.42±0.02 |
0.52±0.04 |
0.48±0.07 |
0.87±0.02 |
||
1000 |
0.49±0.05 |
0.48±0.07 |
0.54±0.10 |
0.75±0.13 |
Table 5 Macrobenthos indices around two cage culture in the southern Caspian Sea (Mean±SE)
However, the difference in macrobenthos indices values was significant during sampling period in two marine farms. Also, in investigating the trend of change in each biological index were determined by highest computational values related to Simpson index in August and the lowest values of other indicators in August (P>0.05)(Table 6).
Marine Farm |
Sampling Course |
Shannon |
Margolov |
Simpson |
Kelarabad |
January |
0.66b |
0.34ab |
0.64b |
March |
0.85a |
0.36ab |
0.48c |
|
May |
0.82ab |
0.39a |
0.53c |
|
August |
0.36c |
0.28b |
0.82a |
|
Abbasabad |
January |
0.91a |
0.63ab |
0.47b |
March |
0.82a |
0.65ab |
0.51b |
|
May |
0.79a |
0.70a |
0.54b |
|
August |
0.39b |
0.54b |
0.79a |
Table 6 Comparison of the average of each macrobenthos bio-index during sampling under the influence of fish farming in two cage culture
®Latin letters in each column indicate a significant difference between each biological index by cage culture in sampling period at 5% level and under Duncan test.
The effect of fish farming and the focal relationship between biotic and non-biotic data
The results showed that there was a focal correlation between biotic and non-biotic factors around cage culture in Klarabad and Abbasabad regions with a linear relationship under RDA testing (Figure 5).
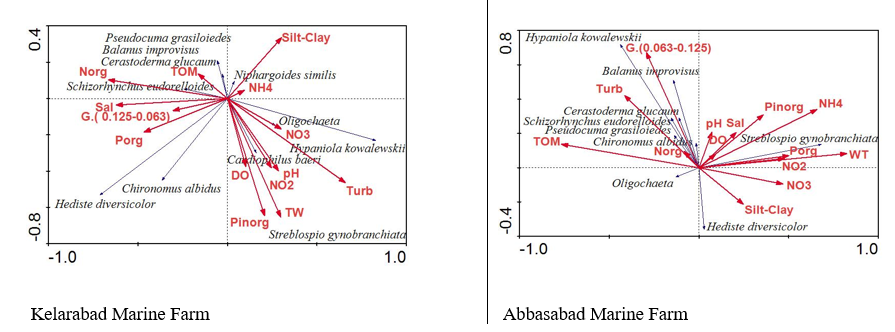
Figure 5 The effect of fish farming in cages on the relationship between environmental and high density of benthic invertebrates around cages in Kelarabad and Abbasabad in the southern Caspian Sea (2014-2015) (TW, water temperature; Turb, turbidity; Sal, salinity; DO, dissolved oxygen; pH, acidity; NO2, nitrite; NO3, nitrate; NH4, ammonium; Norg, organic nitrogen; Porg, organic phosphate; Pinorg, inorganic phosphate; TOM, total organic material; Silt-Clay, G, grain size (0.063-0.125).
Usually, the environmental impact assessment of fish farming being assessed focuses on some of the standards for water quality and biological organisms. Since each of the two offshore farms in this study had four cages, it is considered small-scale aquaculture for marine culture. However, Environmental Assessment (EIA) surveys are performed entirely on large-scale projects in marine fish cage culture. This study has increased awareness of environmental issues related to marine aquaculture in the Southern Caspian Sea region and can be used for better management of the marine environment. In fact, this study is a Strategic Environmental Assessment (SEA) model,20 and the focus were more on measuring water quality, sedimentation and change of macrobenthic communities around offshore fish farms and the method used is common in many countries.20 It is essential to assess the environmental impact, model the transport capacity, and effluent effects of the area. Furthermore, in most countries, there are no methods to assess these factors accurately or there are no necessary skills for aquaculture in the current situation, but the process of environmental assessment activities is improving based on human needs. Although fish culture in sea cages brings risks to the environment, but good marine farm management minimizes these risks.21,22
Usually, the effects of fish cage culture on water quality are on the turbidity, dissolved oxygen, organic material, nitrogen, and total phosphorus23 presence. If the location of the cage is chosen correctly, these effects are reduced to 30 meters from the sea cages.22 Because the soluble nutrients of nitrogen and phosphorus compounds are easily consumed by phytoplankton and large marine algae.24
In this study, changes were observed in the physics and chemical factors of water around each site were subjected to periodic changes and no significant effect on fish farming at distance from the cage and in different directions. However, the concentration of these parameters was somewhat evident at distances close to the site (up to 50 m). The average water flow velocity, using the data in the articles and reports of the National Oceanographic Research Institute and the Fisheries Organization of Iran25 is more than 10-15 cm per second in the south of Caspian Sea. These currents in the central region of the southern Caspian Sea fluctuates from the sea level with a flow of 26 cm/s to a depth of 30 meters about 7 cm/s. In the range of cage establishment from sea level to a depth of 8 m, water flow rate has been reported of 18-26 cm /s.26 The 26cm/s to 30m depth fluctuates about 7cm/s. Therefore, the possibility of the impact of water currents on the limited change of biological and non-biological parameters around these small-scale farms is near to expectation. In the analysis, physicochemical parameters of water around cage culture in both study areas were determined, nitrogen and phosphorus compounds in the main components 1, 2 and 3, considering 67% of the total variance, were included as parameters to be effective (Table 3). Increased nutrients in the environment around fishponds may not only be affected by aquaculture activity, but also by specific physical characteristics of the area such as water change conditions and litter dynamics or because of local eutrophication.27
So, there is a possibility that when the thermal layering fails, the nutrients and other compounds that reached the sediments or deeper layers from the cage culture are returned to the water column.28,29 Therefore, the effect of fish farming in the environment around the cage depends on factors such as rearing management and hydrological conditions of the area. Large, excreted particles or food not consumed by fish, quickly settle, and accumulate in sediments and substrate of sea.30 The sedimentation of the substrate sediment around the fish farms in the two marine farms was silt clay particles and then sand. The difference between the percentages of sediment granulation in the geographical direction relative to the cages was more significant than that of the distance and the sampling period to the cage at the two sites. Therefore, in this study, the percentage of sedimentary tissue depended on the environment and the location of the fish cage and was independent of the effect of fish culture activity.
A review of past sources shows the high share of silt-clay grains in sediment granulation at depths with an average of more than 20 m in the southern Caspian Sea.25,31 Usually, annual changes in the percentage of sand have been reported in other studies in the southern basin of the Caspian Sea, which depends on the amount of sediment inflow into the Caspian Sea and the rate of sedimentation. In the present study, the percentage of very fine sand grains increased significantly in May in all stations. In this increase, a regular trend was observed, so that this increase was more in the south direction in the stations farther from the cage. Due to the long distance of the sampling areas from the coast and the low impact of river flows on the change of substrate sediment texture, the possibility of the impact of sea currents is possible. In this study, the total organic material of the substrate in both sites outside the rearing period had minimal values in August. However, the highest measured values were related to January and before the start of rearing and during the fish farming period. During the rearing period, the maximum values were related to March. Therefore, its changes cannot be considered exclusively because of fish farming. In some studies, it has been suggested that seasonal changes in the amount of organic material in the surface layer of sediments are integrated with the effects of fish farming.32
Macrobenthos in marine ecosystems play a vital role in the water cycle of water, metabolism, pollution, and by-products discharge.33 Habitat, long-life cycle, high species diversity with different sensitivities to environmental stresses and their important role in the food cycle between sediments and water are some of the advantages of using Macrobenthos communities to assess the quality of aquatic ecosystems.34 Any change in the environment on benthic organisms may cause irreparable damage to their communities35 and various ecological conditions such as depth, temperature, season, salinity, dissolved oxygen, zinc and pH amount of organic material and granulation of substrate sediments that effect their distribution and diversity.36 In this study, it was observed that no significant difference between the density and biomass of macrobenthos at the two sites during the sampling period, and the highest percentage of macrobenthos density at both sites belonged to the other category, especially Streblospio gynobranchiata (excluding fish farming period) in January and August and Hypaniola kowalewskii in March and May (during rearing fish). On the other hand, macrobenthos Streblospio gynobranchiata has been the dominant species in the previous two decades until now in the southern Caspian Sea.25,31 Therefore, the change in the dominance of this species in the cage area during the fish rearing period indicated its effect on fish farming. 25,31 Also, there was a linear relationship between macrobenthos and environmental factors under RDA testing, with differences between the two sites. In this study, the commonalities of this relationship are mentioned. Data showed that the temperature factor was the most important factor in increasing the density of the dominant species in both sites. Dominant species were affected by temperature, salinity, pH, dissolved oxygen, nitrogen, and phosphorus minerals. In general, the natural conditions of the environment prevailed over the change in the environmental factors around the fish cage culture.
This study therefore showed the general conditions of the Caspian Sea in the emergence of predominant macrobenthos species. On the other hand, in contrast to the dominant benthic species, most species with low density were affected by organic material and organic nitrogen and in contrast to Streblospio gynobranchiata during the rearing period (March and May) with an increase in organic material and organic nitrogen compounds. Both sites had the lowest density compared to outside the rearing season (January and August). In a study, Rebecca et al.,13 showed using principal component test (PCA) with 76% of the changes and reductions in the invertebrate abundance were associated with distance from the cage and chemical variables, which had the highest effect up to 15 m far from the cages. However, it can be seen from the results of this study that the effect of fish farming on macrobenthos was not significant due to the small-scale size of the fish farming sites and due to proper ventilation of water in the cage settlement area. Compared to control stations (distance 1000 m) more was affected by seasonal changes and ecological conditions. In other words, it can be said that in general, it is the effects of small-scale fish farming that are difficult to detect due to the rapid dilution of large-scale waste32,37 In a similar study, Bascinar et al.,38 evaluated the effects of aquaculture in cages on benthic communities in the Black Sea despite the dominance of Capitella sp. Fish farming activity was rated low and without negative effects and was believed to effect the organic materials due to the dispersion of rearing waste as a result of water flow from , the distance from shore and proper depth of cages establishment (25-50 m). By placing cage culture at lower depths, it is possible for the waste to be easily dispersed to areas away from the cage.39 Therefore, this could be effective in reducing the effects on fish farming in the cage. Although fish farming in these two sites was done on a small scale, it may also be possible that due to the location of the cages (about 5 km from the shore and a depth of 30 m with good water flow) minimize the effects of fish farming on the environment around the cage.
None.
Author declares there are no conflicts of interests.

©2023 Farabi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.