Journal of
eISSN: 2378-3184


Research Article Volume 6 Issue 4
1Director, Marine Science Association (MSAM), Myanmar
2Marine biologist and Research Diver in Marine Conservation Programme in Fauna & Flora International (FFI), Myanmar
3Director, Fauna & Flora International (FFI), Myanmar
Correspondence: U. Soe-Htun, Director, Marine Science Association, Myanmar (MSAM), Yangon, Myanmar
Received: April 24, 2017 | Published: November 1, 2017
Citation: Soe-Htun U, Maung A, Mon S, Ha ST, Aung ST et al. (2017) Biodiversity, Distribution and Coverage of Seagrasses in the Myeik Archipelago and Rakhine Coastal Areas, in Myanmar. J Aquac Mar Biol 6(4): 00164 DOI: 10.15406/jamb.2017.06.00164
Seagrasses provide ecological services to marine organisms that contribute towards food security and economic benifits to local communities living around their meadows. Surveys on seagrass taxonomy, distribution and extent were carried out in Myanmar in 14 sites within the Myeik Archipelago and along the Rakhine coast to assess the status of these important habitats. This study follows the guidelines of taxonomic monographs and the SeagrassNet manual to survey species diversity, percent cover and extent. A total of 11 species of seagrasses were recorded, including Syringodium isoetifolium, Cymodocea rotundata, C. serrulata, Halodule uninervis, H. pinifolia, Enhalus acoroides, Thalassia hemprichii, Halophila beccarii, H. decipiens, H. ovalis and H. major. Of these, Halophila pinifoliawas the most commonly observed species and the only one to be distributed across all 14 study sites. In contrast, Halophila beccarii was only recorded at Ma Gyi along the Rakhine coast. Other uniquie species distributions include Halophila major which was solely found along the Rakhine coast and H. ovalis which was exclusively encountered along the Myeik Archipelago. The habitat types of seagrasses between the two Coastal Regions were also found to be differred with seagrass meadows in Taninthayi most commonly observed in coastal intertidal habitat whereas those in Rakhine were recorded in the fringing reef and deep subtidal habitat. In terms of species diversity among the 14 study sites, Ma Gyi Gyaing and Pho Htaung Gyaing showed the highest in Rakhine with 9 species each while Zar Det Ngye I (East) and Pa Law Kar Kyan I in Taninthayi contained 7 species each. Highest percentage cover of seagrass meadows was observed at Maung Shwe Lay Gyaing, in Rakhine with 67.00% and the highest coverage in Taninthayi at Lampi I (East) with 64.57%. Given the environmental services provided by seagrasses their protection within Myanmar is critical. All areas should be granted a certain level of protection although priotiry firstly needs to be given to Ma Gyi Gyaing in Rakhine given its species diversity, being the only site to conatin the Vulnerable listed Halophila beccarii species and beacsue of its high percent cover. Such an area should be gazzetted as a Marine Protected Area (MPA) along with Pa Law Kar Kyan I., Zar Det Ngye I. (East) and Lampi I. (East) in the Taninthayi Coastal Region and Ma Gyi Gyaing, Pho Htaung Gyaing and Maung Shwe Lay Gyaing in the Rakhine Coastal Region given their species diversity and extent.
Keywords: Biodiversity, Conservation, Ecological accounts, Morphology, Local distribution, Myanmar, Percent cover, Rakhine Coastal Region, Taninthayi Coastal Region, Seagrasses taxonomy
Seagrasses are a relatively small group of submerged flowering plants of approximately 72 species, representing less than 0.1% of the angiosperm taxa growing in shallow coasts of the tropical and subtropical regions. However, about 60% of seagrass meadows globally have been seen reductions in their distribution since 1980.1,2 The ecological importance of seagrass beds has been well documented and includes the provision of sheltered habitats and crucial feeding, spawning and nursery grounds for economically important species of marine invertebrates and fish species.3-7 Furthermore they are key primary producers, involved in epibenthic and benthic production; provide important nutrients and contaminant filtration, producers of oxygen, recycles of nutrients.8 However, since 1980 about 60% of seagrass populations globally have seen a reduction in their distribution due to habitat destruction and marine pollution.1,2
Seagrasses occur all along three Coastal Regions of Myanmar, namely Rakhine, Ayeyarwady Delta and the Gulf of Mottama (Martaban) and Taninthayi. Ten species of seagrasses has been described in Myanmar and include, viz., Syringodium isoetifolium (Ascherson) Danty, Cymodocea serrulata (R. Brown) Ascherson et Magnus, C. rotundata Ehrenberg et Hemprich ex Ascherson, Halodule uninervis (Forsskal) Ascherson, H. pinifolia (Miki) Den Hartog,9 Enhalus acoroides (Linnaeus f.) Royle, Thalassia hemprichii (Ehrenberg) Ascherson, Halophila beccarii Ascherson, H. decipiens Ostenfeld and H. ovalis (R. Brown) Hooker f.10-15 Their importance to Myanmar fishers is well known with local people calling seagrasses Leik-Sar-Phat-Myet, meaning the food of marine turtles. In addition, the seagrass meadows are known to serve as important feeding grounds for the sea cow, Dugong dugon which is recognized as endangered species under the IUCN Red list. Given their importance, both ecologically and economically, and the global decline in segarss beds, the protection of seagrasses within Myanmar is seen as paramount. The objective of this study was to know the updated information on the current status of biodiversity, distribution and coverage of seagrasses at select sites within the Taninthayi and the Rakhine Coastal Regions of Myanmar. This information will be used to guide the creation of Marine Protected Areas within Myanmar to ensure such habitats are conserved and used for long term monitoring of seagrasses.
Ten study sites were selected in the Myeik Archipelago along the Taninthayi coast (Figures 1 & 2) and four study sites in Rakhine (Figures 1 & 3) to gain an understanding of their status and suitability for MPA designation. Surveys were conducted in 2015 between the 6th of March to 4th of April in Tanintanryi and 1st to the 31 May and included the following sites:

Figure 1 Map showing 14 study sites with the biodiversity and distribution of seagrasses growing in the Taninthayi and Rakhine Coastal Regions: 1. Zar Det Gyi; 2. Zar Det Ngye (West); 3. Zar Det Ngye (East); 4. Pa Law Kar Kyan I. (St Luke I.); 5. Nyaung Wee I.; 6. Bo Cho I.; 7. Lampi I. (East); 8. Lampi I. (West); 9. Taw Wet I. (South); 10. Taw Wet I. (North); 11. Ohn Kyun I.; 12. Ma Gyi Gyaing (Shwe Thaung Yan); 13. Pho Htaung Gyaing; and 14. Maung Shwe Lay Gyaing.
Abbreviations Si, Syringodium isoetifolium; Cr, Cymodocea rotundata; Cs, C. serrulata; Hu, Halodule uninervis; Hp, H. pinifolia; Ea, Enhalus acoroides; Th, Thalassia hemprichii; Hb, Halophila beccarii; Hd, H. decipiens; Ho, H. ovalis and Hm, H. major
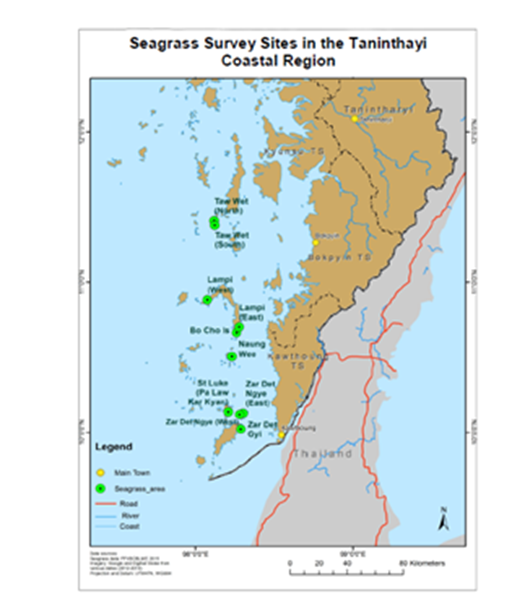
Figure 2 The survey sites of seagrass areas in the Myeik Archipelago, in the Taninthayi Coastal Region.
Taninthayi coastal region:
Zar Det Gyi I.
Seagrass bed was located in front of the mangrove communities at Lat 10.02003º, Long 98.28963º. Substrates are muddy sand and sandy mud to offshore. The area of seagrass bed was 32.80 ac. The percent cover of seagrasses along the cross-transects was estimated on March 23, 2015.
Zar Det Ngye I. (West)
Seagrass bed was located in front of the mangrove communities at Lat 10.11687º, Long 98.28199º. Substrates are muddy sand near shore becoming sand to offshore. The area of seagrass bed was 12.10 ac. The percent cover of seagrasses along the cross-transects was estimated on March 22, 2015.
Zar Det Ngye I. (East)
Seagrass bed was located in front of the mangrove communities at Lat 10.1251º, Long 98.3045º. Substrates are muddy sand inshore and sandy mud at offshore. The area of seagrass bed was 19.90 ac. The percent cover of seagrasses along the cross-transects was estimated on March 24, 2015.
Pa Law Kar Kyan (St. Luke)
Located in front of the mangrove communities at Lat 10.13461º, Long 98.21011º. Substrates are muddy sand nearshore and sandy mud to offshore. The area of seagrass bed was 83.00 ac. The percent cover of seagrasses along the cross-transects was estimated on March 21, 2015.
Nyaung Wee I.
Seagrass bed was located in front of the mangrove communities at Lat 10.50319º, Long 98.23227º. Substrates are muddy sand in nearshore, becoming sandy mud to offshore. The area of seagrass bed was 46.40 ac. The percent cover of seagrasses along the cross-transects was estimated on March 20, 2015.
Bo Cho I.
Located in front of the sandy beach without mangrove communities at Lat10.66216º, Long 98.26º. Water depth 5m. Substrates are muddy sand in nearshore and sandy mud to offshore. The area of seagrass bed was 382.50 ac. The percent cover of seagrasses along the cross-transects was estimated on March 18, 2015.
Lampi I. (East)
Seagrass bed was located in front of the sandy beach without mangrove communities at Lat 10.70202º, Long 98.27984º. Substrates are muddy sand and sandy mud to offshore. The area of seagrass bed was 216.70 ac. The percent cover of seagrasses along the cross-transects was estimated on March 19, 2015.
Lampi I. (West)
Seagrass bed was located in front of the sandy beach without mangrove communities at Lat10.88089º, Long 98.07436º. Substrates are muddy sand nearshore and sandy mud to offshore. The area of seagrass bed was 10.60 ac. The percent cover of seagrasses along the cross-transects was estimated on March 16, 2015.
Taw Wet I. (South)
Seagrass bed was located in front of the mangrove communities at Lat 11.37642º, Long 98.12234º. Substrates are muddy sand becoming sandy mud to offshore. The area of seagrass bed was 28.90 ac. The percent cover of seagrasses along the cross-transects was estimated on March 11, 2015.
Taw Wet I. (North)
Seagrass bed was located in front of the mangrove communities at Lat 11.40776º, Long 98.12032º. Substrates are predominantly muddy sand and becoming sandy mud to offshore. The area of seagrass bed was 45.50 ac. The percent cover of seagrasses along the cross-transects was estimated on March 11, 2015.
Rakhine Coastal Region:
Ohn Kyun I.
Seagrass bed was located in front of the sandy beach without mangrove communities at Lat 16.388765º, Long 94.229125º. Substrates are muddy sand in nearshore and sandy mud to offshore. The area of seagrass bed was 17.30 ac. The percent cover of seagrasses along the cross-transects was estimated on May 8, 2015.
Ma Gyi Gyaing
Seagrass bed was located in front of the sandy beach without mangrove communities at Lat 17.072122º, Long 94.451406º. Substrates are muddy sand nearshore and sandy mud to offshore. The area of seagrass bed was 39.83 ac. The percent cover of seagrasses along the cross-transects was estimated on May 1, 2015.
Pho Htaung Gyaing
Seagrass bed was located in front of the mangrove communities at Lat 17.170547º, Long 94.491739º. Substrates are muddy sand in nearshore and sandy mud to offshore. The area of seagrass bed was 94.22 ac. The percent cover of seagrasses along the cross-transects was estimated on May 2, 2015.
Maung Shwe Lay Gyaing
Seagrass bed was located in front of the sandy beach without mangrove communities at Lat 18.305367º, Long 94.329312º. Substrates are muddy sand in nearshore and sandy mud in offshore. The area of seagrass bed was 23.79 ac. The percent cover of seagrasses along the cross-transects was estimated on May 15, 2015.
Fresh and live materials of seagrasses growing in the natural beds of 14 study sites were sampled by uprooting the seagrasses with a small trowel or knife from 6-3-2015 to 31-5-2015. Snorkeling or scuba equipment was used for the submersed habitats of seagrasses growing in subtidal zone of the Rakhine coast indepths between 2-5m, while surveys were conducted during the ebb tide in the Myeik Archipelago. The collections were initially washed, cleaned and preserved in 10% formalin in seawater. Samples of seagrasses were examined mainly on the vegetative characters with a dissecting microscope, and then pressed on herbarium sheets to prepare as voucher specimens for each locality. As for taxonomic account, all specimens were identified using the standard monograph of seagrasses prepared by den Hartog & Kuo et al.9,16 This study had followed the classification system used by Fortes.17 All voucher specimens were deposited at the Herbarium of Department of Marine Science, Mawlamyine University (MMB), Mawlamyine, Myanmar.
In relation to the ecological accounts, this study has followed the SeagrassNet protocol by Short et al.,18,19 consisting of three fixed, parallel, 50 m cross-transects referred to as cross-transects A, B and C, with cross-transect A closest to shore and C most seaward; B, midpoint of these cross-transects were established on a transect laid out seaward, perpendicular to the shore. Percentage cover of seagrasses was visually estimated within 12 randomly placed0.25m2 quadrats along each cross-transect using a photo guide of percent cover. As noted above these works were done intetidally in the coastal areas of the Myeik Archipelago (Figure 4A) but subtidally in the Rakhine coastal areas using snorkling or scuba equipment (Figure 4B). Positions and areas of seagrasss for each study site were recorded by GPS with extent being recorded by walking around the seagrass bed taking GPS points every 10 secs. The physical parameters, namely temperature using a mercurial thermometer, salinity using a refectometer, water depths using handheld sounder were measured in the field. The types of substrate were also recorded.
In the present study, a total of 11 species of seagrasses were identified: including Syringodium isoetifolium (Ascherson) Danty, Cymodocea rotundata Ehrenberg et Hemprich ex Ascherson, C. serrulata (R. Brown) Ascherson et Magnus, Halodule uninervis (Forsskal) Ascherson, H. pinifolia (Miki) den Hartog, Enhalus acoroides (Linnaeus f.) Royle, Thalassia hemprichii (Ehrenberg) Ascherson, Halophila beccarii Ascherson, H. decipiens Ostenfeld; 10. H. ovalis (R. Brown) Hooker f. and H. major (Zoll.) Miquel (Table 1). A detailed account of the taxonomy of these plants including identification guide, ecological accounts and supplementary survey data were shown in Appendices 1-8 of this report. Of these species, only one, Halophila beccarii is considered Vulnerable according to the IUCN RedList, with all other species listed as Least Concern.
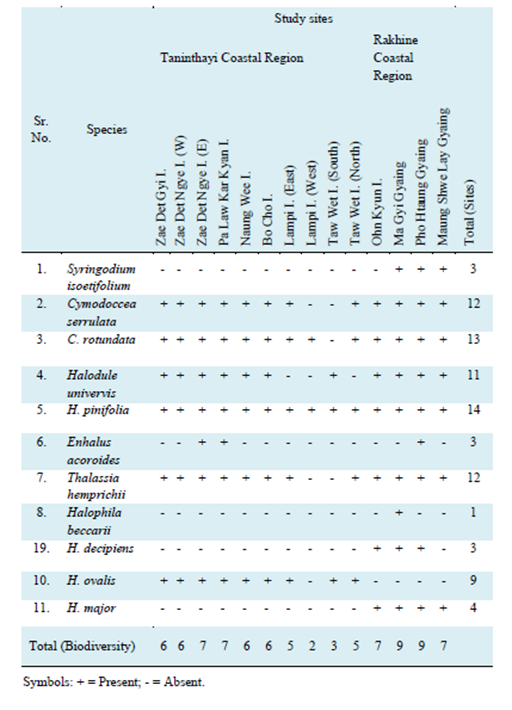
Table 1 Biodiversity of seagrasses distributed in 14 study sites in the Taninthayi and Rakhine Coastal Regions of Myanmar.
Syringodium isoetifolium(AschersonDanty (Figure 5)
Cymodocea rotundataEhrenberg et Hemprich ex Ascherson (Figures 6-7)
Cymodocea serrulata(R. Brown) Ascherson et Magnus (Fig. 8)
Halodule uninervis(Forsskal) Ascherson (Figure 9)
Halodule pinifolia(Miki) den Hartog (Fig. 10)
Enhalus acoroides(Linnaeus f.) Royle (Figure 11)

Figure 11 Enhalus acoroides: A. Habit, B. The natural bed, C. Male flower (arrowhead), D. Female flower(arrowhead) and E. Fruit (arrowhead).
Thalassia hemprichii(Ehrenberg) Ascherson (Figure 12)
Halophila beccarii Ascherson(Figure 13)
Halophila decipiens Ostenfeld (Figure 14)
Halophila ovalis(R. Brown) Hooker f. (Figures 15)
Halophila major (Zoll.) Miquel (Figure 16)
In this study, Halophila pinifolia was the most commonly observed species and the only one to be distributed across all 14 study sites (Table 1). In contrast, Halophila beccarii was only recorded at Ma Gyi Gyaing along the Rakhine coast. Other uniquie species distributions include Halophila major which was found along the Rakhine coast and H. ovalis was encountered only along the Tanintharyi coast. The most frequently observed species along the transects was Cymodocea rotundata and H. ovalis while the least was Thalassia hemprichii. Enhalus acoroides and Halophila beccarii were not recorded along the transects and only opportuniscally observed during the survey and considered low in abundance.
The tidal habit of seagrasses between the two Coastal Regions was also found to differ with seagrass meadows in Taninthayi most commonly observed in intertidal zones whereas those in Rakhine were recorded in the subtidal zone (Table 3). In terms of species diversity among the 14 study sites, Ma Gyi Gyaing and Pho Htaung Gyaing showed the highest in Rakhine with 9 species each while in Taninthayi Zar Det Ngye I. (East) and Pa Law Kar Kyan I. contained 7 species each. Highest percentage cover of seagrass meadows was observed at Maung Shwe Lay Gyaing, in Rakhine with 67.36% and the highest coverage in Taninthayi at Lampi I. (East) with 64.57% (Table 2) (Figures 17.-18). Although no statistical tests were undertaken no clear pattern was observed in terms of species diversity or density from inshore to offshore.
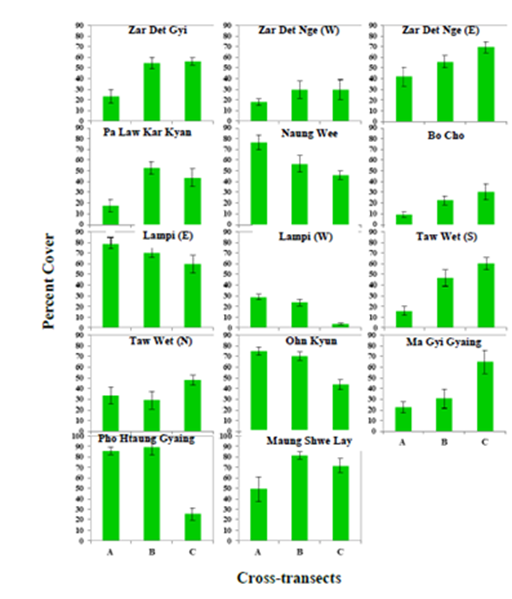
Figure 17 The percent cover of seagrasses along 3 cross-transects in 14 study sites. Abbreviations: A, B and C- Cross-transects.
The current study was able to develop an easily replicable baseline for 14 seagrass sites within Myanmar to allow for long term monitoring of seagrass beds and provide the ability to quanitatively measure the impact of management interventions aimed at seagrass conservation. Of the 14 sites surveyed, only four had been previously studied using the same methodology,14 which were located in the Tanintahyi Region, and so comparisions can be made. Of the four only one, Lampi I. (East) showed an increase in percentage cover with 64.57% recorded in the current survey compared to 45% in the 2007 surveys.14 A number of reasons maybe responsible for this increase such as a decrease in shrimp and fish catch in the area leading trawlers to search elsewhere for catch or from the increase in presence of Department of Forestry staffs at Marine National Park headquarters in the Lampi Island opposite to the seagrass bed, resulting of greater support by the the NGO community to the MPAs management. Two of the other sites, Taw Wet I. (North) and Nyang Wee I., did show a decrease in percentage cover but only by 9% and 11% respectively, with such a result potentially down to transect placement. These sites will however need to be monitored to ensure that its only statistical errors causing this decrease and not anthropoengic impacts such as bottom trawling. The final site, which had previously been surveyed in 2007, was Lampi I. (West) and this seagrass bed has seen an extensive loss in percentage cover with 18.75% recorded in this survey compared to 80% cover in 2007. Boat activity in this area was observed to be quite high during the surveys with this part of Lampi Island providing protection for many boats during periods of high winds and as such could be targeted by trawlers when conditions away from the island are unfavourable. These seagrass beds were noted to have a high cover of sand sediments smothering their stems. The current support being provided to manage this Marine National Park by organisations such as the Italian NGO Oikos may help to ensure this seagrass beds protection and long term conservation.
In the present study, however, unlike Kress20 no specimens of Zostrea marina were recorded. This species normally occurs in temperate waters and known to extend into the higher latitudes of Myanmar waters and has previously been found in all three Coastal Regions of Myanmar. Further surveys are therefore needed to eludciate the status of this species within the country.
Seagrass meadows were mostly in intertidal zone encountered in front of the mangrove communities in Tanintharyi whereas those in Rakhine are commonly found in the subtidal zone in front of the sandy beaches (except for the seagrass meadow Pho Htaung Gyaing located behind the mangrove swamp). For this reason, the luxuriant growth of seagrasses was observed in all coastal areas of Rakhine due to moderate and favourable environmental parameters but not in coastal areas of the Myeik Archipelago of Tanintharyi Coastal Region due its natural habitat in the interdal zone under heavy environmental stresses (Table 3). In the present study, other uniquie species distributions include Halophila major which was solely found along the Rakhine coast21 and H. ovalis which was exclusively encountered along the coastal areas of the Myeik Archipelago.
Although Soe-Htun et al.12 reported there were no stresses in the meadows of seagrasses in coastal areas of Myanmar, with these ecosystems showing pristine and climax conditions; they are now facing the problems such as smothering by sand as noted above. Such issues can arise from trawlers stirring up sediments or from land-slides where forest areas have been cleared such as those observed on Zar Det Gyi I. In general, seagrass beds in Myanmar are exposed to a number of threats including runoff from cities and towns and hazardous wastes and oil dispersals released from industrial zones located in the upper areas of natural seagrass beds are seen as serious threats to these habitats. Bottom trawlers also operate directly through seagrass beds targeting shrimps and other marine species destroying these habiats. Smoothering of seagrasses in sediments from sand mining operations in the Myeik Archipelago resulting in reduced ability of seagrasses to photosynthesize.
Management actions are therefore required immediately to ensure these habitats are not lost which would have deveastaing consequences for both the aquatic environement and for people’s livelihoods. However given the limited resources available in Myanmar to manage all these areas focus must be steered towards those sites which could be considered key biodiverse areas. Therefore, to prioritise the most important sites and focus management inteverntions a simple ranking system was developed for the surveyd areas using uniqueness (in terms of species representation), species richness and percentage seagrass cover (Tables 1-4). Although all sites should receive some level of protection six sites stood out in terms of the above paramters with Ma Gyi Gyaing (39.83 ac) in Rakhine consider the most important site being 1) one of the most diverse, 2) the only site to conatin Halophila beccarii, the most threatened of all the 12 species recorded with a ranking of Vulnerable under the IUCN Redlist, and 3) because of its high percentage cover. Moreover, in terms of species diversity of seagrasses, Ma Gyi and Pho Htaung Gyaings showed the highest in the Rakhine Coastal Region with 9 species while Zar Det Ngye (East) I. and Pa Law Kar Kyan I, represented higest in the Myeik Archipelago waters including 7 species of seagrasses (Tables 1). Highest percentage cover of seagrass meadows was observed at Maung Shwe Lay Gyaing, in Rakhine Coastal Region with 67.36% whereas the lowest was at Lampi I. (West) with 18.75% (Tables 2), (Figures 17-18). Other sites worthy of immediate protection include Pho Htaung Gyaing (94.22 ac), Zar Det Ngye I. (East)(19.90 ac), Pa Law Kar Kyan I. (St. Luke I.)(83.00 ac), Ohn Kyun I. (17.30 ac) and Maung Shwe Lay Gyaing (23.79 ac) (Tables 3). As a first step, these sites should be provided some level of protection either as strict no-take MPAs or has carefully managed gear restricted areas with a strong emphasis on bottoms trawlers and other gears with may negatively impact the seagrass.

Table 4 Seagrass sites ranked according to uniqueness, species richness and percentage cover with 1 being the highest priority for protection and 5 the least. Shaded boxes indicate priority sites
Such processes can however take time and require human capacity and various other resources to manage such interventions. As such a number of required actions have been recommended which are seen as necessary for effective management of seagrasses in Myanmar and designed to guide decision makers in developing conservation plans for seagrasses to ensure these habitats are protected as follows:
A total of 14 study sites- ten study sites, viz., Zar Det Gyi I, Zar Det Ngye I (West), Zar Det Ngye I (East), Pa Law Kar Kyan I (St. Luke I), Naung Wee I, Bo Cho I, Lampi I (East), Lampi I. (West), Taw Wet I (South) and Taw Wet I (North) in the Myeik Archipelago of the Taninthayi Coastal Region and four study sites, namely Ohn Kyun I., Ma Gyi Gyaing, Pho Htaung Gyaing and Maung Shwe Lay Gyaing in the Rakhine Coastal Region, were surveyed to assess the current status of seagrasses growing along the coastal areas of Myanmar. Eleven species of seagrasses were recorded from the Myeik Archipelago and Rakhine coastal areas of Myanmar, including Syringodium isoetifolium, Cymodocea rotundata, C. serrulata, Halodule uninervis, H. pinifolia, Enhalus acoroides, Thalassia hemprichii, Halophila beccarii, H. decipiens, H. ovalisand H. major. Taxonomic key to the species of seagrasses collected from the two study areas in Myanmar:
Of the 11 species of seagrasses collected in this study, only one, Halophila beccarii is considered Vulnerable according to the IUCN RedList, with all other species listed as Least Concern. Halophila beccarii was only recorded at Ma Gyi Gyaing. Of these species, Halodule pinifolia was the most commonly observed species. Ma Gyi Gyaing, being the only site to conatin the Vulnerable listed Halophila beccarii species and beacsue of its high percent cover, should be gazzetted as a Marine Protected Area (MPA) along with Pho Htaung Gyaing, and Zar Det Ngye (East) given their species diversity and extent.
The first author, U. Soe-Htun is very grateful to the late Dr. U. Min-Thein, Director (Retd), Microalga Biotechnology Department, Myanmar Phamaceutical Factory (MPF), Yangon, Myanmar for invaluable guidance in the studies of seagrasses since his surveys in Maung Shwe Lay Gyaing in 1980. We are indebted to Robert Howard, FFI Myanmar‘s Marine Conservation Programme Manager, for his useful assistance during this study. We would like to express our sincere thanks to the local people who kindly help us in many ways during our field trips. Many thanks go to U Thaung Htut and U Zaw Lin Tun, Staffs of Marine Science Association, Myanmar (MSAM) for their helpful assistance in the preparation of the manuscript. Funding for this work from the the Bay of Bengal Large Marine Ecosystem (BOBLME) Project “Seagrass Monitoring in Myanmar”, under a joint project with Fauna & Flora International and BOBLME of the Food and Agriculture Organisation (FAO) of the United Nations, is most appreciated.
None.

©2017 Soe-Htun, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.