Journal of
eISSN: 2378-3184


Research Article Volume 7 Issue 1
1Biology Department Campus University of Voutes Greece
2Foundation for Research and Technology Institute of Electronic Structure and Laser Greece
Correspondence: Glaropoulos Alexis Biology Department Campus University Vouton Greece, Tel 30 2810 394060
Received: February 14, 2017 | Published: January 18, 2018
Citation: Glaropoulos A, Papadakis V, Kentouri M (2018) Behavioural Differences in Tank-Held European Sea Bass under Dark/Light Environment. J Aquac Mar Biol 7(1): 00179 DOI: 10.15406/jamb.2018.07.00179
The study investigated the behavior of European sea bass (Dicentrarchus labrax), in laboratory-scale conditions and controlled light environment. Fish were confined in tanks that had been previously split into two equal compartments. A tear on the net allowed fish to freely moved between the two compartments. An extra net frame, placed above tanks altered light environment to: (1) Illuminated Holding Area – Shadow Escape Area, (2) Shadowed Holding Area - Illuminated Escape Area and 3) Illuminated Holding Area - Illuminated Escape Area, as control condition, for a period of 13 days. Sea bass showed a strong preference to the illuminated compartment of the tank, from the beginning of the trials. Crossings to the shadowed compartment were regular but always followed by a reverse action to the illuminated area. In addition, they occurred in series of 7 individuals. Reverse crossings to the initial shadowed compartment were rarely observed throughout the experiment, even if fish had to suffer starvation. Behavioural results regarding sea bass light preference may have essential implications in marine biology and aquaculture, including species domestication as well as manipulation via artificial light.
Keywords: Fish behaviour; Illumination; Crossing; Dicentrarchus labrax; Light preference
Light is an important abiotic factor that has been associated with fish biology and behaviour. Most fish depends on their vision system to perform activities such as foraging Meager & Luchiari et al.1,2 breeding. Particularly, biorhythms have been related to feeding time, either in nature or aquaculture as well as under restricted feeding conditions.3 In recent years, artificial light has been introduced in both fisheries4 and aquaculture5 towards attraction to specific areas and population guide for manipulation and avoidance of unfavorable conditions.6,7
European sea bass (Dicentrarchus labrax) is a commercial interest species in the Mediterranean,8 with several studies involved into behavioural variations due to light alterations. Up to now, such studies have been mostly involved feeding behaviour towards on-demand technology9,10 growth and survival of larvae.11 In parallel, fisheries studies have not clarify species preference to certain light intensities.4 Sea bass tended to continuously swim around a light source, but neither attracted nor inhibited by the light source. Light spectra may also have an influence on sea bass response to light attraction7 which has already been proven for Atlantic salmon to specific spectra of submerged artificial light.12
Preference for a certain environment indicates fish ability to discriminate in between different conditions13,14 via specific behavioural strategies. Such studies have been widely performed in a variety of farmed species in a way to promote animal welfare. Particularly, studies in caged Atlantic salmon 15 and cod16 have already shown that this species can alter its vertical distribution looking for optimal conditions but also, it can be vertically guided in the cage environment via artificial light.17 Such studies demonstrate the potential of artificial light to promote fish welfare,6 disease avoidance18 and sexual maturation.19 Mediterranean aquaculture lacks of such studies that may put one step further on a more sustainable industry.
Such studies highlight the significance of light on culture protocols that promote fish welfare and growth performance. Indeed, though feed still remains available at the bottom of the tanks, feed utilization under dark/light environment is yet to be examined. Moreover, the effect of light should be further investigated into offshore farming facilities, where limited visual contact to pellets through the water column might be caused of low illumination level.20
The present study investigated (1) the preference of this species to illuminated or dark environment and (2) whether light condition is linked to feed utilization in experimental conditions. The experiment was performed in tanks under fully controlled conditions, where fish were able to express preference between illuminated and shadowed areas. Such studies are essential, since they promote welfare conditions for early domesticated species in aquaculture. However, they could further suggest light as another option to manipulate Mediterranean species in any field of the aquaculture production, a widely used technology in Atlantic salmon culture.4,5,25
Experimental set up
The study was conducted at the aquaculture facilities at the University of Crete, Greece. Nine laboratory-scale parallelogram tanks (100lt) were stocked with 15 size-matched (length 115 cm, depth 40 cm, width 34 cm) European sea bass (Dicentrarchus labrax) each. Fish were left to adapt the tank environment for a period of seven days, under food satiation condition sat 14.00 every day. Photoperiod in the experimental area was set to 12L: 12D by means of two 30 W fluorescent tubes positioned 30 cm from the upper side of the tanks. Lamps were not dimmed but switch on/off in a fixed time period.
Prior to any experiment, all tanks were split into two equivalent compartments (50 l) by a removable net pen (white polypropylene net, 17 mm hexagonal mesh opening) fixed in a plastic frame (31 x 28 cm) that was fitted tightly to the bottom and the two sides of the tank. A tear (5.1 cm height) located centrally on the net pen, allowed fish to cross between the compartments of the tank. All fish group were held to the left safe zone of the tank, where food was always provided. The other tank compartment was the escape area, with no food access. Light involved plastic, black colour frame that was placed above tanks to differentiate them in three (x3) combinations of illumination conditions 1) Illuminated Holding Area (IHA) - Shadowed Escape Area (SEA), 2) Shadowed Holding Area (SHA) - Illuminated Escape Area (IEA) and 3) Illuminated Holding Area (IHA) - Illuminated Escape Area (IEA) as control condition. Light intensity in every tank compartment was measured with a Lux meter (Extech Instruments, L825251), and was 330 ± 7 lux in the illuminated area and 8 ± 2 lux in the shadowed area. Sanchez et al.3 The experiment lasted 13 days, where all fish could freely swim within the tank compartments through the net tear.
Fish swimming activity
Sea bass swimming activity was observed via nine external colour digital CCD cameras (Fire-i, Unibrain), placed in front of each tank, recording continuously from 8.00 until 20.00 o’clock daily.22 The cameras’ acquisition rate was set to 9 frames per second (fps). Night observation was no performed since the parameter under consideration (illumination condition) was not valid during the night.
Fish and light condition
All acquired video data were analyzed with the use of custom-made software,23 written in Lab View (National Instruments). The analysis was focused on the crossings through the net tear - both from the holding to the escape area and reversely, the duration of each event and the time spent on each compartment of the tank.
The first experimental day was fully analyzed and the exact number and time of all crossings were reported. Fish reacted to the sudden change in light intensity, after having spent seven days of acclimatization period, where no light level occurred in tanks. This analysis was restricted only to the first day, no fish remained in the SHA by the end of this day. We assume that learning was neglible during this first day.
After the first day, sea bass were mostly aggregated to the illuminated areas of each tank. Therefore, for the remainder of the experimental days (day 2 to 13), a different approach was followed for the analysis of behavior. Five time periods of 15 minutes each within the day (starting at times: 9:00 early morning - 11:00 morning - 13:30 before feeding time - 15:30 after feeding time - 18:00 late afternoon) were selected so as to analyze crossing activity under different illuminated areas and thus species preference to light.
Water quality variables
Water temperature (24 ºC), salinity (38ppt) and oxygen saturation (> 85%) remained stable during the experiment. In addition, the concentrations of nitrite and ammonia were less than 0.3 mg l-1 and 1.5 mg l-1 respectively.
Statistical analysis
Data analysis statistics performed in SIGMASTAT software (statistical package; Systat Software, San Jose, Calif). Fish were not individually tagged or recognizable. Data were treated group-wise (n=3). Overall crossing activity during the first day between light conditions and within tanks was assessed via two-way ANOVA. A three-way ANOVA for the remaining 2-13 days determined differences in crossing activity for each light condition, tank and time period within the day. T-tests evaluated differences in crossing activity between the five time periods of the day. Normality tests (Shapiro-Wilk test) were performed so as to control the distribution of the data. All Pair wise Multiple Comparison Procedures were tested using Tukey Test. Pearson correlation tests examined differences on growth performance indexes at different light conditions. The level of significance was set at P < 0.05. For all mean values, standard error (SE) was calculated.
European sea bass response to the experimental design
Sea bass crossings occurred in all tanks. However, differences in the illumination level were linked to species behaviour in tanks and particularly on the time spent at each tank compartment. When both light conditions (light/dark) existed in tanks, fish distribution significantly altered between tank compartments (Figure 1). Fish from IHA- group slowly and continuously passed in and out the illuminated area but mostly (in duration and time) remained to the illuminated tank compartment. This arose from the 50% of the population that remained in this compartment by the end of the first day. The first passage to the dark compartment occurred on this group, approximately five minutes that the experiments started. In contrast, sea bass from SHA- group gradually (within 4 hrs) moved to the illuminated area of the tank and remained there up to the end of the day (Figure 1). In general, sea bass responded to different illumination level by moving on the illuminated compartments.
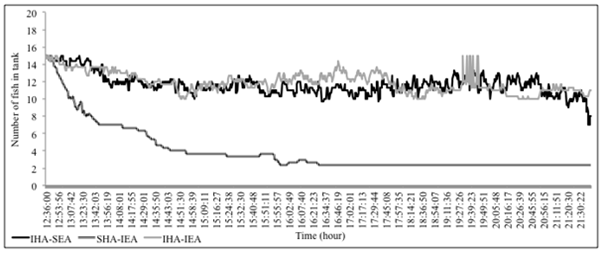
Fish crossings differed between light conditions (q > 14.7, P < 0.05), with over 80% of SHA- group population had moved to the IEA compartment. During the first experimental day, sea bass showed an equal distribution among the two compartments in the control condition, with only few crossings in and out the illuminated area. In fact, this group was mainly distributed in the bottom of the tanks, formatting a strong schooling behaviour. There was neither a change in fish distribution before and after the feeding time (Figure 1). A clear pattern was easily observed on fish activity that involved crossings in series of two up to seven individuals. Mean crossing duration was 0.87 ± 0.18 seconds.
The effect of altered light condition on sea bass crossing behaviour
Under different illumination level in tanks (days 2-13), number of crossings was higher (P < 0.05) from illuminated to shadowed compartments (18 crossings 15min-1) as compared to the reverse condition (2crossings 15min-1), but always followed by the reverse crossing to the illuminated area (Figure 2a). Crossings did not significantly differ within the day (10 crossings 15min-1, on average), but they were twice higher (P < 0.05) after the feeding time (Figure 3a). Time interval between in and out of the illuminated area was measured to be 8 seconds, on average. Significant differences were also found between the second and third day (F > 2.23, P > 0.05) as compared to the remaining experimental days (Figure 2a). In general, fish in IHA- group were mostly aggregated (80 % of the population) to the illuminated area of the tank by the end of each experimental day. Conversely, crossing activity was almost absent (<3 events day-1) in the SHA- group during the study (Figure 2b). Though food supply continued at the SHA- group regardless the population level, crossings rarely observed until the fifth day (Figure 2b), but fish were all aggregated to higher illumination level (IEA). Random tests on video recording at the feeding time revealed remains of uneaten pellets at the bottom of the tank that clearly confirmed fish unwillingness to return to the shade. Some crossings started more frequently after the sixth day, while were mostly occurred after feeding time (Figure 3b), indicating weakened light preference at food deprivation. However, crossing activity up to the end of experiment remained significantly lower compared to the relative at IHA- group and control group (Figure 2b). Correlation analyses between growth performance indexes and light condition indicated higher SGR (r = 0.5, P < 0.05) and FCR (r = 0.2, P< 0.05) index at IHA- group. Ultimately, the control condition maintained a strong schooling behaviour inside the tanks, while fish performed crossings during the experiment (Figure 2c) but also particularly (q> 0.09,p < 0.05) after feeding time (Figure 3c). During the experiment, fish distribution was even (55-45 %) between the two compartments of the tank.
The present study indicates preference of tank-held European sea bass to illuminated areas. Sea bass preferred light than dark environment, either remaining or moving towards there. However, the continuous crossing activity from the illuminated to shadowed areas of the tank suggests that the motivational strength to light preference might be subjected to innate species behaviour, regarding species biology24 and particularly remarkable swimming activity.25 Sea bass light preference might open new discussion on species-specific behaviour during domestication period.
The results of the first experimental day clearly demonstrate the significance of light as an abiotic driver to sea bass swimming behaviour. Sea bass from IHA-group presented a bimodal distribution pattern, moving in and out the dark compartment of the tank during the study. Both tank exploitation was necessary against undesirable fish density or species biology that is characterized by an elongate body size 24 and a remarkable swimming activity.25 Intense crossing activity had also been described in laboratory-caged sea bass 26, where crossing in series of several individuals were observed through the net tear. The continuous crossing activity during the study is likely to either search for food or tank exploitation, while comes in agreement with similar results in fisheries study with other species4 where mackerels, tuna as well as other species tended to move in and out of the illuminated field driven by feeding motivation. Significantly higher number of crossings after the feeding time in all fish groups might suggest that fish swimming and feed intake is unaltered from the illumination level. In the present study, sea bass mostly mainly aggregated to the illuminated compartment of the tank. However, the presence of the net pen and the small volume of the tank might have resulted to a more complicated behaviour.
The sudden creation of shade on the SHA-group is likely to act as an acute stimulus that increased crossing activity, which also coincides with the previous acclimatization period of fish under the same light environment. The decision to instantly move towards illuminated areas of the tank was probably driven by the intense schooling behaviour, which is well-known species characteristic27 that ensures lowered predation, energetic advantages and favorable conditions. Sea bass individuals from this group were not motivated to reverse crossings in the dark compartment, even though they have to starve. Uneaten pellets that were regularly present at the dark left bottom of the tanks further indicated fish preference to higher illumination level. Sea bass avoidance to lower illumination level should be further investigated in commercial-scale aquaculture, since light overruling to feed could be deleterious for a sustainable aquaculture.
Other motivational factors, which are also known to overrule fish behaviour in tanks, are for example, food supply 28 and fish density.22 Indeed, food deprivation in such studies resulted to different behavioural strategies from Atlantic halibut and sea bream, probably driven by search for food. Though, in such studies, sea bream required up to15 days29 to alter its behaviour, in the present study sea bass initiated a few crossings in the shadowed compartment after 5 days. The intense crossing particularly at the feeding time food suggests that the motivational strength of food might be unaltered by light condition.
In conclusion, the present study indicates that sea bass exhibit light preference in tank environment. In addition, under laboratory conditions, light might overrule feed utilization. Nevertheless, future research should be focused on different light intensities or light regimes and the potential behavioural effect on caged sea bass. For example, studies in fisheries have already shown species attraction by shorter wavelength lights4. In parallel with fisheries, related studies have already shown promising results for salmon manipulation17 and pathogen avoidanc5 through guidance in different swimming depths.
Instant light preference of tank-confined European sea bass might have further implication to commercial-scale aquaculture via submerged fixed or moving artificial lights. Controlling caged sea bass population using artificial lights, based on species preferences, could provide efficient operations tool that promote fish welfare and general growth and production.
This study has been financially supported by the Commission of the European Communities under the Seventh Research Framework Program "Food, Agriculture and Fisheries and Biotechnology, Area 2.1.2 Increased sustainability of all production systems (agriculture, forestry, fisheries and aquaculture); plant health and crop protection", 226885 "PREVENT ESCAPE - Assessing the causes and developing measures to prevent the escape of fish from sea cage aquaculture". The authors would like to thank the Hellenic Center for Marine Research (HCMR), especially its director, Dr. Pascal Divanach for providing us the sea bass and Dr. Ioannis E. Papadakis, Eleftheria Georgiou and Angeliki Antonakaki for helping us on the experiment, the analysis of the videos and the extraction of the biological data.
None.

©2018 Glaropoulos, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.