Journal of
eISSN: 2378-3184


Research Article Volume 2 Issue 2
1Department of Environment and Agriculture, Curtin University, Bentley, WA, Australia
2Department of Agriculture and Food South Perth, WA, Australia
3Department of Fishery, Hasanuddin University, Km 10 Makassar Indonesia 90225
Correspondence: Irfan Ambas, Sustainable Aquatic Resources and Biotechnology, Department of Agriculture and Environment, Curtin University, Bentley, WA, Australia, Tel +61 -8- 92664508
Received: December 01, 2014 | Published: April 25, 2015
Citation: Ambas I, Fotedar R, Buller N (2015) Bacillus mycoides Improves Health of Gastrointestinal Tract in Marron (Cherax cainii, Austin 2002). J Aquac Mar Biol 2(2): 00023. DOI: 10.15406/jamb.2015.02.00023
The present study examined the health status of the gastrointestinal tract (GIT) in marron after a two-month feeding trial with a diet supplemented with host origin, Bacillus mycoides. Two groups of marron were fed with either a basal diet or probiotic added at 108cfu/g of feed. Microbial density, microvilli length and number, intestinal epidermis layer morphology and hepatopancreas indices of weight and moisture content were evaluated. Supplementation with B. mycoides in marron feed significantly improved intestinal bacterial density (4,007±121 million cfu/g of GIT) compared to basal diet fed marron (723.7±45.2 million cfu/g of GIT). Microvilli density (per 100 µm2) was also significantly higher (10.50±0.25 µm) compared to 5.71±0.24 in basal diet fed marron. Significantly higher villous length (4.93±0.11 µm) was observed in probiotic fed compared to basal diet fed marron (3.91±0.18 µm).
The intestinal epidermis layer of probiotic fed marron showed increased folding and thickness compared to basal diet fed marron. Higher hepatosomatic indices (Hiw) and low moisture content (HM%) in probiotic fed marron indicated efficient functioning of the healthy gut. The present study suggests that supplementing host origin customised probiotic in feed improves gut health as measured by microbial density, microvilli length and number, intestinal epidermis layer and hepatosomatic indices.
Keywords: Probiotic, Microbial density, Microvilli, Hepatosomatic indices, Marron
BA, Blood Agar; Cfu/g, Colony Forming Unit Per Gram; Cm, Centimetre; GIT, Gastrointestinal Tract; GLL, Glycerol Lab Lemco broth; H & E, Hematoxylin and Eosin; Hiw and Hid, hepatosomatic wet and dry; µm, micrometre; HMDS, Hexamethyldisilazane; (HM%), Moisture Content; L, Litre; L/min, Litre/minute; M, Molar; MGA, Marron Growers Association; SEM, Scanning Electron Microscope; Wwh, Weight of wet hepatopancreas; Wdh, Weight of dry hepatopancreas; Wt, Total Weight of Marron
It is widely established that in addition to skin and gills, the gastrointestinal tract (GIT) is considered one of the major routes for pathogenic invasion in aquatic animals.1-4 Therefore, study of the GIT of aquatic animals as a physical and immunological barrier is increasingly important, and it is accepted that digestion and immunity are complicated physiological processes that have co-evolved.4,5 The GIT of aquatic animals plays an important role in non-specific immune defences, as it provides an initial barrier to pathogen entry.5,6-10 The first step in bacterial invasion of the intestine is mediated by adhesion of pathogenic bacteria to mucosal surfaces and disruption of the microbial balance.7,11 In most fish hatcheries, intestinal microbial disorders caused by bacterial disease are considered to be a major cause of mortality, thus stability of the intestinal microbes and gut health are essential for the health of an organism.10 As a result, much attention has been focused on the development of probiotics in order to maintain a stable, beneficial gut microbial population.12 Study of morphology and intestinal health of aquatic animals using prebiotics has been evaluated in red drum, Sciaenopsocellatus,13,14 rainbow trout, Oncorhynchus mykiss,15 gilthead sea bream, Sparusaurata,16 tilapia, Oreochromis niloticus,17 channel catfish Ictalurus punctatus,18 Pacific white shrimp, Litopenaeusvannamei,19 marron Cherax tenuimanus20 and its combination with probiotic.4,21-24
Although the use of probiotics in aquatic animals has been reviewed by many authors,7,25-30 information for probiotics effect on intestinal health and morphology is extremely limited.18,31 To date, probiotic effects on intestinal health and morphology have been studied only in Nile tilapia, Oreochromis niloticus,32 seabream, Sparusaurata L.31 and beluga, Husohuso.33 Bacillus mycoides is a bacterium found in marron and the environment that has favourable probiotic properties including growth inhibition of V. mimicus and V. cholerae non-01, is susceptible to a majority of antibiotics, non-pathogenic to marron, produces a wide range of enzymes34 and improved the immunity and health of marron.35 The aim of the present study was to examine the effects of Bacillus mycoides on intestinal health and morphology in marron36 as determined by bacterial density, hepatopancreas indices including moisture content, microvilli density and length, and histological examination of intestinal cells.
Culture system, experimental animal and feed preparation
The experimental units were cylindrical plastic tanks (80 cm diameter, 50 cm high and 250 L in capacity). The tanks were filled with freshwater and supplied with constant aeration, and sufficient number of marron shelters of PVC pipes with appropriate diameters. Each tank was also equipped with a submersible thermostat set to 24°C and a recirculating biological filtration system. The water in the tank was recirculated continuously at a rate of approximately 3 L/min. To maintain good water quality in the tanks, water exchange at a rate of 10-15% of the total water volume was performed twice a week, after siphoning out the faeces and uneaten feed. Marron (weight 33-65g) were obtained from the Marron Growers Association (MGA) in Northcliffe and Manjimup, Western Australia. The 250 L tanks were stocked with marron at a density of 12 marron/tank. Before commencement of the experiment, marron were kept for two weeks in the experimental tanks for acclimation. During the two month experimental period, a commercial pelleted diet (26% protein, 47-50% carbohydrate, 9% fats and 8.9% ash) from Enviroplus Pty Ltd., Perth Australia was fed to marron at a rate of 1.5 % body weight per day. Bacillus mycoides was isolated from a number of healthy marron.
The isolate was identified by the Bacteriology Laboratory, Animal Health Laboratories, Department of Agriculture and Food, Western Australia, using a matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometer (Bruker Bioscience Corporation), Vitek Compact II (Biomerieux) and conventional biochemical methods according to standard procedures and identification methods.37 Subsequently, the strain was suspended into 1 mL aliquots of GLL (Glycerol Lab Lemco broth) and stored at - 80°C. Supplementation using the probiotic strain was performed as per Hai and Fotedar.21 In brief, prior to probiotic supplementation of the experimental diet, a pure culture of B. mycoides was grown on 5% horse blood agar (BA) plates for 24h at 25°C. Colonies in logarithmic phase of growth were emulsified in sterilised distilled water and serially diluted. The optical reading of each serial dilution was recorded, and a viable count performed to obtain a standard curve for inoculum density. From the standard curve, the amount of the diluted probiotic was calculated to achieve the desirable supplementation density of 108 colony forming unit (cfu) per gram of feed. The pellets were air dried, packed and stored at 4°C until used.
Data collection
At the termination of the experiment, the GIT health status of marron was determined through analysis of bacterial density, microvilli length and density, histologic assessment of GIT epithelium, and moisture content and weight of the hepatopancreas. All animals used for analysis from both treatment groups were of equal in weight or length size in order to minimise misinterpretations due to size variations.
Bacterial density: The bacterial density of marron GIT after feeding with probiotic supplemented feed was measured at the beginning and end of the experiment. Ten marron from each treatment group were sacrificed by placing them at -20°C for 5 minutes before aseptic removal of the GIT. The marron dorsal shell was cut-off horizontally from tail to head until the hepatopancreas and intestine were exposed. The hepatopancreas was removed, placed in a sterilised pestle, weighed and then homogenised. Similarly, the intestine from individual animals was collected aseptically and homogenised with a micropestle in a 1.5 ml microfuge tube. The homogenised hepatopancreas, and the homogenised intestine were serially (10-1, 10-2, 10-3, 10-4, 10-5 and 10-6) diluted. Fifty microliter of each serial dilution was inoculated onto a BA plate and incubated overnight in a CO2 incubator at 25°C. A colony count was performed for each dilution to determine the total number of aerobic bacteria.
GIT microvilli assessment by micrograph: The distal part of the marron intestine was observed using a scanning electron microscope (SEM) following an established method.38,39 The intestinal tract of five marron from each treatment group was dissected and immersed in 3% glutaraldehyde in 0.1M cacodylate buffer overnight. Following overnight immersion, the GIT was washed in 3 change softhe cacodylate buffer and3 changes in distilled water for 5min per change. The intestine was immersed in 2% OsO4 for 2h followed by 3 washes in distilled water for 5 min per wash. Dehydration of the sample was performed through solutions of 50%, 75%, 95% ethanol for 5 min per solution and finally 3 times in100% ethanol for 5min per change followed by chemical drying by washing in a series of 50%, 75% and 100% (twice) hexamethyl disilizane (HMDS) in ethanol solutions for 5 min per change. The final stage involved drying the samples at room temperature, mounting on as tub using carbontape and then coating with gold before viewing the samples under a pressure scanning electron microscope (LX30). The images obtained from SEM were used to describe villous height and density (number per group surface area) in the GIT. The height of microvilli (µm) was measured following established methods.31,32 At least 10 villous per section were randomly selected and measured using a computerised morphometric technique. The height of each villous was measured from the villous bottom to the tip, and the average height of these 10 villi was expressed as the mean villous height. Villous density (villous/100 µm surface area) and villi per group were counted according to Sang and Fotedar.39
Histological assessment of the intestine: Histological preparation and assessment of marron GIT post-feeding with probiotic and basal diets were prepared by Animal Health Laboratories, Department of Agriculture and Food Western Australia. Five marron GIT from each treatment group were dissected and fixed in 10% buffered formalin for 24h. Dehydration of the tissue was performed by passing through a series of 70%, 85%and 98%alcoholsolutions.The samples were vacuum embedded in paraffin. The histological sections of 4-5µm was stained with Hematoxylin andeosin (H&E). The sections were examined and photographed using an Olympus BX50 microscope.
Hepatosomatic indices (HiW): The hepatosomatic indices (Hiw) of marron fed with probiotic supplemented diet and basal diet were calculated as per established equations.40,41 In brief, the hepatopancreas of ten marron from each treatment group were removed placed in foil and weighed. For hepatopancreas moisture content, the hepatopancreas was dried at 110°C for 24 h. The results, expressed as wet hepatosomatic indices (Hiw), dry hepatosomatic indices (Hid) and hepatopancreas moisture content (HM) were calculated as follows;
Where;
Hiw: Wet hepatosomatic indices (%)
Hid: Dry hepatosomatic indices (%)
Wwh: Weight of wet hepatopancreas (g)
Wdh: Weight of dry hepatopancreas
Wt: Total weight of marron (g)
HM: Hepatopancreas moisture content (%)
Data analysis
Data were analysed using SPSS statistical program version 22. Comparison of the mean values using T-test was performed to determine significance and the results were presented in tables 1-3 and graphs.
|
Ingredients |
Percentage (%) |
|
Wheat Flour |
49.35 |
|
Fish Meala |
33.78 |
|
Soybean Meal |
10.15 |
|
Fish Oilb |
3.2 |
|
Wheat Starch |
1.85 |
|
Betainec |
1.20 |
|
Cholesterol |
0.25 |
|
Premixd |
0.15 |
|
Ascorbic Acid |
0.05 |
|
Calcium Carbonate |
0.02 |
|
Total |
100 |
Table 1 Ingredients of the basal diet
All ingredients were supplied by Specialty Feeds Pty Ltd WA, Australia.
aPeruvian fishmeal, 56 % CP
bCod liver oil
cBetaine anhydrous 97%
dCommercial vitamin and minerals premix for trout
|
Parameters |
Probiotic Diet |
Basal Diet |
|
Villous Height |
4.93±0.11b |
3.91±0.18a |
|
Villous Per Group |
10.50± 0.25b |
5.71±0.24a |
|
Villous Density |
20.28±0.70b |
13.93±0.4a |
Table 2 Mean ± SE (n=5) of villous height (µm), villous number per group and villous density (per 100 µm2) of marron hindgut fed probiotic supplemented diet and basal diet
|
Treatment |
Hiw (%) |
Hid (%) |
HM (%) |
|
Basal diet |
6.20 ± 0.02a |
2.71 ± 0.06a |
62.16 ± 1.09a |
|
Probiotic diet |
7.11 ± 0.34b |
3.32 ± 0.25b |
53.43 ±1.68b |
Table 3 Mean ± SE (n=10) of hepatosomatic indices (%) and moisture content (%) of marron fed basal and probiotic supplemented diet.
*Mean value in the same row having different superscript indicates significantly differentat P<0.05.
*Mean values in the same column with different superscript indicate significantly different (P< 0.05)
Hiw: hepatosomatic indices (wet)
Hid: hepatosomatic indices (dry)
HM: Hepatopancreas moisture content
Results
Bacterial density: The mean bacterial density in marron intestine fed probiotic supplemented diet was significantly increased (4007±121million cfu/g of GIT) compared to the bacterial density in the gut of basal diet fed marron (723.7 ± 45.2 million cfu/g of GIT). The diversity of the bacterial population was greater in the GIT of probiotic supplemented diet fed marron compared to basal diet fed, as observed by colonial morphology on BA plates after 24h incubation at 25°C (Figure 1).
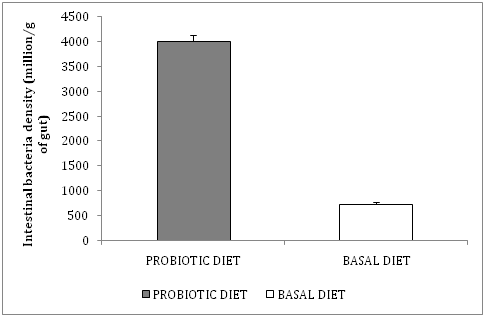
Figure 1 Mean ± SE (n=10) of intestinal bacteria population (million/g of gut) of marron fed basal and probiotic supplemented diet.
GIT microvilli assessment by micrograph
The morphology of marron intestines after feeding with probiotic supplemented diet compared to basal diet is shown in Figure 2. The density and length of the microvilli per GIT surface area was significantly higher in marron fed B. mycoides supplemented diet (A) than microvilli of basal diet fed marron (B). The average density of microvilli per group (number of villous in a row) of marron fed the probiotic diet was 10.50 ± 0.94 compared to 5.71 ± 0.91 in basal diet fed marron (Figure 3).
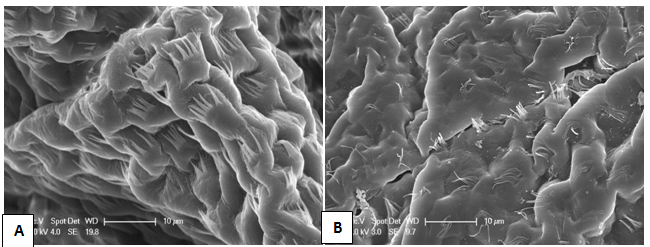
Figure 2 Scanning electron microscopy micrograph of marron hindgut fed probiotic supplemented diet (A) and basal diet (B). (x=2500. Bar=10 μm). In the probiotics fed marron there is an increase in folds and the villi are longer and more numerous.
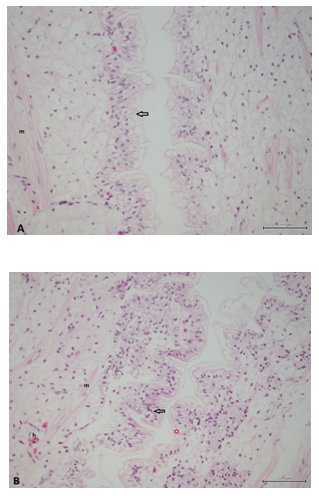
Figure 3 Histological sections of the hindgut from marron fed the control diet (A) and the probiotic supplemented diet (B). The epidermal cells (open arrow) in the probiotic supplemented group are larger and have a more foamy appearance. In both treatments there is some shrinkage artifact of the cuticle from the epithelial cells (red star). Muscle cells (m) and mixed populations of haemocytes (h) can be seen in the lamina propria in both the control and probiotic supplemented animals. Bars indicate 200µm.
Histological assessment of intestine: Histologically there were no major differences seen in the hepatopancreas of marron from the different treatment groups. However, the foregut and hindgut of the probiotic fed marron had more folds and fewer haemocytes compared to the marron fed the control diet without probiotics.
Hepatopancreas weight: Hepatosomatic indices (Hiw) of probiotic fed marron were significantly higher (7.11 ± 0.34) compared to basal diet fed marron (6.20±0.02). In addition, hepatopancreas moisture content (HM%) was lower (53.43±1.68 %) in probiotic fed marron whereas in basal diet fed marron it was 62.16±1.09%.
Discussion
Balcazar et al.42 Zhou and Wang43 suggest that probiotics act in several ways: Firstly by maintaining and restoring normal intestinal microbiota and gut homeostasis; secondly by contributing to the competitive exclusion of bacteria44-47 and thirdly by acting as a source of nutrients and enzymes.7,48-52 Most authors suggest that probiotics of host origin are more favourable compared to other sources as is believed that autochthonous bacteria are able to colonise, multiply and remain predominant in the same host.7,10,25,53,54 The health status of the GIT is most likely determined by the microbial balance of indigenous microbiota49,55 with the density and diversity of bacteria in the intestine having the most impact on intestinal health.56 The main parameters commonly used to assess GIT health in aquatic animals are intestinal bacterial density and diversity, microvilli height and number, gut epithelium, hepatopancreas size and digestive enzyme activity.4,15,31,36,39,56-58
In the present study marron fed a diet supplemented with Bacillus mycoides had improved intestinal morphology, increased density of bacteria in the intestine and a heavier hepatopancreas. All of these features suggest that the marron benefited from the probiotic supplementation. A number of studies show intestinal bacteria density increases after probiotic supplementation which is thought to be due to a probiotic of host origin providing a favourable environment for the indigenous intestinal bacteria. In shrimp Penaeusmonodon, the number of intestinal bacteria increased by up to 803% after supplementation with three Bacillus species (B. pumilus, B. sphaericus, and B. subtilis) isolated from the host.59 In grouper Epinepheluscoioides potentially beneficial bacteria were stimulated, whereas some potentially harmful strains such as Staphylococcus saprophyticus, were suppressed after supplementation with probiotic Psychrobacter sp.60 Reduction of either diversity or quantity of the indigenous microbiota is likely to reduce the effective barrier mechanism normally provided by the commensal microbiota.11,55
Another feature of a healthy digestive system is the density and length of microvilli. In the present study, supplementation with B. mycoides significantly improved the height and number of villi. Other studies on probiotics had similar findings including one on marron using mannan-oligosaccharide (MOS) diet,20 and others using Lactobacillus sp. in Nile tilapia Oreochromis niloticus,32 Bacillus sp. in European lobster Homarusgammarus L.24 and Pedicoccus acidilactici in rainbow trout.61 Contrary to these findings, observed no improvements in microvilli in rainbow trout fed with Bacillus sp. or Enterococcus faecium supplemented feeds, whereas Cerezuela et al.34 found shorter villi in gilthead seabream (Sparusaurata) fed diets containing B. Subtilise, suggesting that the effect of probiotics on microvilli may not be consistent in all species.
In aquatic animals, longer intestinal villi provide greater absorption ability due to their increased surface area.26,34,35,62 They also provide a larger surface area for bacterial colonisation as was reported for Arctic charr, Salvelinusalpinus. Ringo et al.63 observed large populations of bacteria associated with the villous brush borders, while Hellberg and Bjerkas64 detected the bacteria between the microvilli in common wolfish, Anarhichas lupus (L). In addition, Merrifield et al.65 observed greater bacterial colonization between the folds of the mucosal surface because bacteria could become established and sustained more easily at the base of the villi and between the mucosal folds. Merrifield23 suggested that more dense and regular villi may also play a role in disease prevention by reducing exposure to enterocyte tight junctions in rainbow trout, Oncorhynchus mykiss (Walbaum). Various hepatosomatic indices of marron have been reported in several studies. Jussila66 compared hepatosomatic indices of marron at molt and post-molt at different feeding status and found that the lowest (3.8±0.2 %) hepatosomatic indices (Hiw) of marron was observed in non-fed post-molt marron, followed by fed post-molt marron (5.4±0.3%) and the highest (5.6±0.3%) in fed-intermolt stage marron.
Sang and Fotedar67 observed Hiw of marron fed β-1,3 glucan supplemented diet ranged between 6.35-7.17 %. In the present study Hiw of probiotic fed marron was 7.11 ± 0.34%compared to 6.20 ± 0.02% for basal diet fed marron. The improved intestinal health in the probiotic fed marron most likely resulted in the higher hepatosomatic index of these marron. The hepatopancreas, as the main energy reserve in crustaceans and source of various enzymes, has been used as an indicator of crayfish condition 40,41. A heavier hepatopancreas could be an indication of higher digestive enzyme activities.68 Our previous study also indicated that marron fed B. mycoideshad a larger hepatopancreas especially at day 35.35 B. mycoides produces a wide range of enzymes,34 including many exo-enzymes important for digestion 69 and is a reason why Bacillus sp. have been used widely as probiotics.70
Overall, supplementation of host origin B. mycoides in marron feed improved the health of the marron gastrointestinal tract as indicated by an increase in bacterial density, increased and longer microvilli, thicker intestinal epithelium and higher hepatosomatic indices. Consequently the use of host origin (particularly mucosal inhabitants) strains of bacteria with probiotic properties is recommended as these bacteria are able to maintain microbial homeostasis, are well adapted to the host GIT environment and mucosal attachment, and can protect the epithelium layer from potential pathogens, which in turn preserve optimal function of the gastrointestinal tract.
The authors wish to thank Directorate General of Higher Education (DIKTI) of Indonesia which financially supported this study. Sincere gratitude to Dr. Fran Stephens and Mr. Tai Le from Animal Health Laboratories, Department of Agriculture and Food Western Australia for histological samples preparation and analysis. Mr. Le TrungKy for SEM samples preparation, Mr. and Mrs. Harris for the supply of marron and Mr Albert Cooper for proof-reading of the manuscript.
None.

©2015 Ambas, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.