Journal of
eISSN: 2378-3184


Research Article Volume 11 Issue 1
School of Oceanography, Rio de Janeiro State University, Brazil
Correspondence: Ana Carolina Lustosa Gomes de Campos, Rio de Janeiro State University, Av. São Francisco Xavier, 524. 4th floor, block F, room 4143, Rio de Janeiro, Rio de Janeiro, Brazil
Received: February 09, 2022 | Published: March 14, 2022
Citation: de Campos ACLG, Bastos MP, Fernandes AM, et al. Assessment of the environmental sustainability of cobia fish farm (Rachycentron Canadum) in the bay of Ilha Grande - Brazil and the relationship with benthic macrofauna. J Aquac Mar Biol. 2022;11(1):1-7. DOI: 10.15406/jamb.2022.11.00329
Studies on the sustainability of aquaculture have been carried out around the world. The input of organic matter in the sediment and the influence of current velocity on the deposition of particles produced by fish in captivity can be used to assess the distribution of benthic assemblages below marine farms. Sediment and benthic organisms were collected in April, September and December 2019 and in August and October 2020 in the fish farming area and in two control areas in the Bananal cove in Ilha Grande Bay. The measurement of the speed of local currents was carried out below the net-tank, in periods of 24 days in of April and July 2019. The heterogeneity of the biological data was evident between the collection points, indicating that several factors can influence the distribution of benthic macrofauna. There was no correlation between the values of organic matter and species richness and for organic matter and species diversity. The values of the Marine Biotic Index (AMBI) for the three collection stations were smaller than 1 (one), that is, there was a predominance of species sensitive to pollution and organic enrichment. The marine farm and the control areas were considered an unpolluted environment. The results also indicated that the local currents presented insufficient intensities to disperse the organic matter produced in the marine farm to the control areas.
Keywords: aquaculture assessment, benthos, marine sedimentation, oceanic current
According to estimates by the Food and Agriculture Organization of the United Nations (FAO), the world population is expected to reach 9 billion people in 2050 and, given this expressive and continuous increase, there is a concern about how to manage the environment that provides the essential natural resources for human survival, including food production.1,2
Overfishing has been identified as one of the factors that make fishing unsustainable in the long term.3 Thus, the search for sustainable fishing is emerging in order to minimize environmental impacts, reduce waste and social inequality.4,5
Although promising, marine aquaculture requires some care as does any activity introduced into an environment to which it does not originally belong.6,7 The feed that is not ingested by fish in captivity is one of the major problems of the activity. The waste generated in fish farming ends up being deposited below the cages and the impact generated is more pronounced in the sediment than in the water column.8–10 The deposition of particles on the seabed depends on several factors, such as the speed of the currents11 which, depending on the local hydrodynamics, can be responsible for dispersing the particulate material in the water column.
Benthic organisms have restricted mobility and are therefore sensitive to environmental changes. The excess of organic matter deposited in the sediment can lead to an anoxic environment and thus, possibly reduces the richness, abundance and diversity of benthic species in the substrate.12–14 Therefore, it is essential to understand how the benthic community responds to these substrate changes to assess the possible impacts produced by fish farming.15,16
The economic viability of Cobia cultivation presents itself as a promising activity in Ilha Grande Bay, since the Bananal cove has adequate characteristics for the cultivation of the species and excellent results have been observed in the region.17,18 This activity may become an alternative source of income for coastal communities.19–21
The knowledge oceanographic characteristics of the area, as well as the possible impacts generated by the activity, are important for carrying capacity studies that can help in the expansion of Cobia fish farming in the region.22 This study aims to evaluate the sustainability of the Cobia marine farm in the Bananal Cove inlet by analyzing the response of the benthic community to possible changes in the sediment, considering the deposited organic matter and the role of currents in the remobilization and dispersion of particulate matter.
The results obtained will be fundamental for the knowledge of the dynamics of the local currents, as well as the taxonomic survey of the species contributes to the database and the scientific development in the region. These data can help developing management strategies to ensure the capacity of ecosystems to assimilate the waste produced by aquaculture.
Study area
Ilha Grande Bay (22°50’S and 23°20’S, and 44°00’W and 44°45’W) is located southwest of the southern coast of Rio de Janeiro (Figure 1). This estuarine system is partially mixed with ocean waters that penetrate the bay through the eastern and western ends of Ilha Grande and has part of its freshwater input from Sepetiba Bay.23 The bay shelters eleven conservation units, due to the existing ecosystems and biological communities. The port terminal of Angra dos Reis and the maritime terminal of Ilha Grande Bay are also located within the bay.24 Therefore, in this context, there is a concern towards maintaining the sustainable activities that are developed in the area.25
This study was carried out in the inlet of Bananal Cove (23°11’S - 44°26’W) (Figure 1), which is in the northwest of Ilha Grande Bay. This region has promoted the expansion of aquaculture through on sustainable practices.17,18
Sampling stations
Three sampling stations with similar oceanographic and physiographic characteristics were determined (Figure 1) to evaluate the differences in the distribution of unconsolidated substrate benthic biota and local sedimentary granulometry. The first station was set under the fish farm (CU) located in front of Jaconema Beach (23°06'52.6"S and 44°15'48.2"W) and the two control stations (C1 and C2), to the east of the first one, are located respectively at 23°06'01.5"S - 44°15'29.7"W and 23°06'43.5"S - 44°15'29.2"W in Bananal Cove.
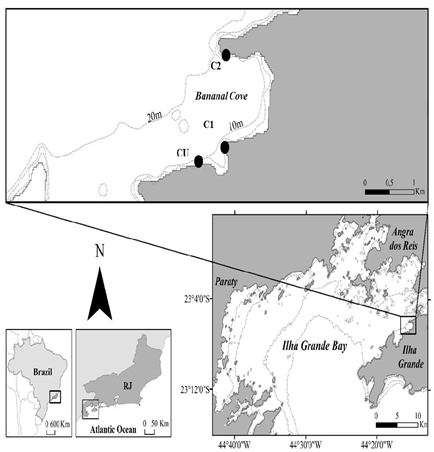
Figure 1 Sampling stations (CU-fish farm, C1-control 1, C2-control2) in Bananal Cove - Ilha Grande Bay, where studies of the benthic community of unconsolidated sediment were conducted.
Theoretical resource
Corrêa-Silva et al.23 adapted an ecological resilience roadmap for coastal regions capable of predicting the use limits of environmental systems, that may be an effective environmental management tool that can be replicated in other coastal zones. Theoretical resources are important for the construction of an environmental resilience assessment script to be adopted as a subsidy to the environmental management and remediation processes and were adopted in the present study. The six steps defined by the authors in the approach to coastal area resilience are as follows.
Circulation of currents
At the fish farm site, current measurements through the water column were obtained using an acoustic Doppler current profiler (ADCP, Acoustic Doppler Current Profiler), 600kHz Work Horse Sentinel model (Teledyne RD Instruments), which registered data in the period from April 4 to 28, 2019 and during the period from June 25 to July 19, 2019. This instrument was deployed with transducers looking up, at 17 meters depth, nearby the buoy of the first tank-net. The sampling frequency was 1 pulse (ping) every 90 seconds and the vertical resolution of the cell was set to 3m.
Quality control procedures were adapted from NOAA, National Oceanic and Atmospheric Administration (2009) and applied to the collected data by means of a computational script written in the Python 3.7 language. In this step, data that present at least one of the following characteristics were considered spurious and discarded: deviations of the Roll and Pitch angles ≥5°; average echo amplitude (AE) ≤30 counts; average magnitude of Amplitude Correlation of transducers (AC) ≤63 counts; percentage of coherence of transducer number 4 (PC4) ≤19 counts; and velocity values greater than three standard deviations (spikes). The correction of the velocity data according to the magnetic declination angle of -22.5⁰ was also performed in this step.
Quantitative analysis of organic matter in the sediment
Five sediment collection campaigns were carried out. Three of them in 2019 (April, September and December) and two in 2020 (August and November). At each of the points (CU, C1 and C2), 5 (five) replicas were collected at a depth of about 15 meters, using the scuba diving technique. The sediment was collected by a cylindrical PVC sampler (core) with 15cm in diameter and 20cm in depth, corresponding to an area of 0.01m². The oxidation of organic matter in the sediment occurred using hydrogen peroxide (H2O2- 30%) according to the classic techniques of sediment analysis.26
Benthic organisms
Collections of benthic organisms were carried out in April, September and December 2019 and also in August and November 2020. The material was collected using the 20cm deep cylindrical sampler described in the previous section. The fauna associated with the sediment was extracted through two sieves of 1 mm and 0.5 mm opening that retained the organisms.27 The macrofauna was quantified and identified, when possible, to the taxonomic level of the species under stereoscopic and optical microscopes (SZ/STMPRO series - Bel Photonics).
Environmental quality index
Macrofauna has been characterized using metrics such as abundance, biomass and diversity. For diversity, the most intuitive and simple measure is the species richness (S), which will be considered the total number of species observed in the community. Of the most commonly used indices, this study will present results for Shannon-Wiener diversity (H'). Another environmental quality index applied was the AMBI. Some authors have already reported the efficiency of this index when applied to the distribution of the benthic community in tropical environments.28
Index based on indicator species
Biological diversity indices - Shannon–Weaver Index (1949)
Species diversity was assessed by the Shannon Index. The index reflects the heterogeneity of a community based on two factors: the number of species present and their relative abundance. Conceptually, it is a measure of the degree of uncertainty associated with the random selection of an individual in the community. The index presents values between 0 and 5 bits. Values equal to five are rare and indicate a high degree of diversity, values close to or equal to zero indicate poor and bad ecological status respectively and, therefore, the diversity of the sample is low. The index is calculated using Equation 1 where pi is the frequency of each species, for ranging from 1 to S (Species richness).
Equation 1
AMBI: AZTI's Marine Biotic Index, or AZTI Marine Biotic Index (BORJA et al., 2003).
It is given by Equation 2, where %G is the representative percentage of each group.
Equation 2
AMBI is based on the proportion of the abundance of five ecological groups correlating them to the degree of sensitivity and to the gradient of environmental stress generated in an environment impacted by organic enrichment.
From samples of benthic organisms, a value between 0 (unaltered substrate), 6 (altered substrate) to 7 (benthos-free zone) is calculated. Organisms range from 0 to 7.0 in terms of the Biotic Index (BI) and ecosystems are classified from Normal to severely polluted.
Data normality was evaluated by the Shapiro Wilk test.29 The tested data were directly determined to be non-normal, therefore, transformers used - log root1, square and arcsine. After the transformation, the test was reapplied, and the p-values were still very low (p<0.005). Thus, the data were not normal, thus a non-parametric statistic was employed. As the tested data were also non-normal, they were selected using the Kruskal-Wallis test29 to compare the collection stations.
Two types of investigation were adopted. In the first one, the data were grouped by campaign, where the H0 hypothesis was that all campaigns have the same characteristics. In the second, the data were grouped by collection station, where the hypothesis H0 was that all stations have the same biological characteristics. When the coefficient p is less than 0.05, the null hypothesis is said to be rejected. To find out the dataset groups with p<0.05, that is, with significant differences, the compared test was applied.
Means of replicates were considered for data analysis. The data were spreadsheets in the Excel 365 package and analyzed in the software version 3.1 (R core Team 2019).
Spearman's correlation consists of understanding how a variable behaves in a scenario where another one is varying, in order to identify whether there is a relationship between the variability of both. The correlation test in this study was used to evaluate how the ecological indices (richness, diversity and AMBI) behave in relation to the organic matter present in the sediment. This correlation measure is evaluated in the range -1 and 1. The closer to the extremes, the stronger the relationship. When the coefficient value approaches 1, there is a positive linear relationship. When the coefficient approaches -1, it is also possible to say that the variables are correlated, but in this case, increasing the value of one variable implies a decrease in the value of the other one. This is called negative correlation. A close to zero correlation coefficient indicates that there is no relationship between the two variables.
Circulation of currents
As displayed in Figure 2, rose plots of the current velocity measurements, obtained in April 2019, revealed a maximum intensity of 10cm/s near the bottom (12m) and 25cm/s in the layer closest to the surface (3m). Velocities below 3 meters present direction predominantly parallel to the local shoreline, approximately 250°, with slightly southwestward and northeastward flows associated with ebb and flood tides, respectively (Figure 3). In the layer closest to the surface (3m), despite the similarities with the deeper layers, more scattered velocity directions and higher intensities are observed which may be due to local wind forcing or the action of surface gravity waves coming from northwest direction. For the July 2019 campaign, displayed in Figure 4, the maximum intensity was 12cm/s in the deepest layer and 35cm/s, near the surface. Overall, the velocity pattern was like that one observed for April 2019 at all depths. In the layer closest to the surface, as observed in April, the intensities and directions were like those observed in the deeper levels, but with more scattered directions and greater intensities.
Figure 4 display tidal residual velocities computed for complete semidiurnal tidal cycles for each one of the two campaigns. Overall, for depths below 3m, values smaller than 2cm/s, with a predominant 250° direction are seen in both campaigns. As mentioned above, this direction is aligned with the orientation of the shoreline in the crop area. At 3m depth, residual velocities show similar magnitudes but different directions between the two campaigns, which may indicate the action of other hydrodynamical forcing along with the tide.
Quantitative analysis of organic matter in the sediment
There were no significant differences between the collection stations regarding the content of organic matter present in the sediment (p=0.1307). However, significant differences in organic matter (p<0.05) were observed in the campaign.
Benthic organisms
Approximately 30.000 organisms were identified (considering replicas). Gastropods with abundance percentages of 76%, 12% of bivalves and 12% of polychaetas.
Among the 34 identified invertebrate taxa, the gastropod class presented the highest species richness (26). The species Caecum brasilicum (Folin,1874) was the most representative (44%) within the group. In addition, the species Finella dúbia (d'Orbigny, 1840) was the only organism observed in all campaigns.
Three species of bivalves were identified: Donax sp. (Linnaeus, 1758); Diplodonta punctata (Say, 1822) and Ervilia concentrica (Holmes, 1860). Diplodonta puncata represented 49% of this group.
The annelids showed low density. The Exogone sp. (Örsted, 1845) represented 48% of the group. The species Perinereis ponteni (Kinberg, 1865); Sacocirrhus sp. (Bobretzky 1872) and Syllis sp. (Lamarck, 1818) have also been identified.
Environmental quality index
AMBI: AZTI's Marine Biotic Index, or AZTI Marine Biotic Index (BORJA et al., 2003).
AMBI values for the three collection stations were <1 and therefore the three collection stations were classified as undisturbed by organic material. This result corresponds to the species within the groups classified in terms of sensitivity to organic matter. The specie Perinereis pontenni was the only representative of group III. From group II, that is, organisms indifferent to organic enrichment, species were identified the polychaete Silllys sp. and Exogone sp., the bivalve Diplodonta punctata and Natica sp. and the gastropod Lampanella minima (Gmelin, 1791), Heminoea elegans (Gray, 1825) and Odostomia sp. (Fleming, 1813).
Control 1 and cultivation presented organisms from ecological groups I, II and III. Control 2 presented only organisms from ecological groups I and II, reinforcing that there was no dominance of organisms linked to a disturbed environment, or species tolerant to excess organic matter. This result is reinforced by the absence or low-density pattern of opportunistic organisms seen earlier.
Campaigns 1, 2 and 3 had the occurrence of Polychaetas of ecological group III, however organisms of this group are characterized by low density and non-significant variation in time, not being considered as an indicative group of ecological impact regarding organic enrichment (Figure 5–7).
Biological diversity indices - Shannon–Weaver Index (1949)
Through the hypothesis test, it can be confirmed that there were no significant differences between the collection stations for richness (p=0.3127) and diversity (p=0.4903).
Spearman's correlation
The correlation between OM and AMBI was equal to 0.733. There were significant differences, p=0.004, for the correlation between the variables. The correlation between OM quantified in the sediment and species richness was weak (Spearman=0.0119). The same was observed for the correlation between OM and species diversity (Spearman=0.0126), with values of p=0.530 (richness) and p=0.947 (diversity), respectively (Figure 8–10).
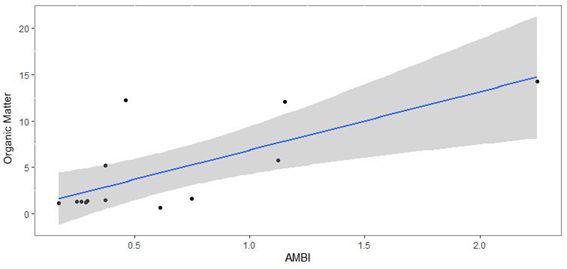
Figure 8 Regression curve between AMBI and organic matter present in the sediment. Darker area around the curve indicates the confidence interval of 95%.
The region where the fish farm is located (area of influence) and the control areas evaluated in this study are characterized as semi-closed, sheltered cove without the presence of river inflows. The current velocity results confirmed the low energy characteristic of the seabed in the Bananal Cove region as verified in previous studies.20,30
High-resolution numerical simulations performed by Rodrigues31 under barotropic conditions and forced only by tides, indicated that an attenuation of tidal current velocities in the Ilha Grande channel region occurs due to the way the tidal wave propagates to the interior of Ilha Grande Bay. The co-tidal maps of the constituents M2 and S2 calculated by the author, from numerical simulations, show that the tidal wave penetrates the west and east entrances of Ilha Grande Bay with a small lag. Thus, the propagation of the tide to the Ilha Grande channel occurs on both sides of the channel and the superposition of these waves attenuates the energy of tidal currents in this region with impacts on the point where the fish farmis located.
Residual velocities are important quantities in environmental studies because they correspond to a permanent drift that results from all hydrodynamic processes that occur in the place. Residual velocities may, therefore, be responsible for the effective transport of sediments and other suspended particles in the water column over a tidal cycle. Residual velocities were calculated for complete cycles of semidiurnal tide during the collection period and presented values below 2cm/s, with a predominant west/southwest direction in both collections. This direction was shown to be aligned with the orientation of the shoreline in the crop area, as commonly occurs for shallow flows along channels and bays. Thus, the transport of fecal pellets and unconsumed feed in the water column is expected to occur predominantly to the west of the point of fish farm. Although, by judging by the low residual velocities, this transport should be expected as small, an assertive assessment of it depends fundamentally on the time of falling particles.30
According to Sanz-Lazaro & Marin9 in studies of fish farming impacts, even in deep places, if the bottom currents are not intense, organic matter will be deposited below the fish cultures, causing changes in the diversity patterns of benthic communities. Ballester-Molltó et al.10 report that particles in the water column are subject to three-dimensional actions and currents may not be the only variables acting on the displacement of particulate matter generated in fish farm. The authors also state that each cultivated species produces fecal pellets with its own characteristics and with different transport densities, so the intensity of the current needed to displace a particle is variable. The results of the present study indicate that the bottom residual currents tend to be directed, to the west (inner part of the beach). The control regions of this study are located northeast of Jaconema Beach and, therefore, it is possible that they are not influenced by material from fish farm. The fish farming activity evaluated in this study did not affect the benthic community differently. This was confirmed by the data on species richness and diversity, which showed little variability between seasons in all campaigns. However, studies of biogeochemical parameters, which would be complementary, were not performed. Apostolaki et al.32 evaluating benthic macrofauna and the possible impacts caused by aquaculture, also did not show differences in the abundance of organisms in the control and cultivation regions in Mediterranean seas.
Authors around the world, who evaluated the benthic distribution below marine farms, reported the occurrence of opportunistic species when evaluating the organic enrichment present in the bottom sediment.33,34 González-Salazar et al.35 and Lima et al.36 evaluated the changes in the assemblage of benthic communities in marine farm areas and identified that increases in polychaete populations are frequent in eutrophic environments with low diversity and species richness. Although the record of organic matter in the unconsolidated substrate is considered one of the factors that favor certain species, the present study did not verify a relationship between the organic matter of the sediment and the distribution of the benthic fauna.
The highest density organisms presented were representative of the gastropod and bivalve classes. These taxa are described by Sanz-Lazaro & Marin9 as the most sensitive to changes produced by oxygen depletion, demonstrating that the organic matter quantified in the sediment is possibly not favoring tolerant and opportunistic species. Bastos25 also verified the predominance of the classes of bivalves in the Bananal cove and there were no reports of opportunistic species in the region, confirming the aforementioned results. Landuci30 who also carried out studies on the marine farm on screen, in the Bananal cove, did not show the occurrence of opportunistic species at the site, despite the abundance of polychaetes and nematodes below the cobia ponds being greater than in the adjacent areas. In addition, even with the verification of the organic contribution to the soil below the crop from leftover feed and fecal pellets, the impacts on the benthic community of unconsolidated substrate were not proved. The author also found abundance percentages, gastropods with abundance percentages of 91%, 7% of bivalves and 2% of polychaetes.
It is worth mentioning that both in the present study and in the previous ones, the taxonomic discrimination at lower levels allowed a more precise analysis regarding the representatives of the benthic community and their role as an environmental indicator.37 Approximately 30 thousand individuals were identified and distributed in 34 taxa.
Borja et al.28 used AMBI to assess benthic environmental quality from values of abundance of organisms in relation to fish farming areas and controls in European regions. The authors described an increase in benthic quality with distance from the marine farm region. Differently, in the present study the values of AMBI (<1) showed that the substrate of the sampling stations is occupied primarily by organisms sensitive to the contribution of organic matter (group I) and, therefore, the greater occurrence of these species in relation to the species of the others groups may indicate that the site is set up in an unpolluted environment. The occurrence of group II organisms, characterized as indifferent to organic enrichment, highlights that there are no local effects on organic matter concentration. The absence of opportunistic species corroborates the results presented regarding the differences between the organisms identified in the fish farm area and in the control areas.
The correlation analyzes were weak or insufficient between the richness and diversity of organisms with the organic matter of the sediment. However, the correlation of AMBI and organic matter was positive. Considering that the index qualifies organism’s sensitive to this variable, this result was already expected. Jayaraj et al.38 evaluating the effects of organic matter on ecological indices applied to benthic fauna, also found no relationship between them.
According to Pearson & Rosenberg12 different local ecological conditions under the effects of fish and fish disturbance range from severe to almost insignificant impacts on the benthic community. Ji et al.11 states that fish farming does not always result in significant changes in the seabed, especially considering the topographical and hydrodynamic characteristics of the site. Papageorgiou et al.39 describe that the impacts on the benthic environment of an unconsolidated substrate are not always easy to detect if there is no predominance of fine sediments with a high content of organic matter.
Hartstein & Rowden40 consider reasonable to expect that the organic matter deposition from a culture of at least 3 years of age is sufficient to cause some difference in the composition of benthic macroinvertebrates. Considering that the fish farm of Cobia in the Bananal Cove has been active and uninterrupted for over 10 years, added to the results presented in this study and in previous studies it is possible to consider that the marine farm on the site is sustainable and the activity is possibly not causing impacts associated with residues produced by fish in the benthic community of unconsolidated substrate.
The results obtained in this study can be affirmed that the OM in the sediment is not enough to show a gradient of organisms below the culture and in the control regions. The velocity of currents on the seabed is like previous studies in the region, demonstrating a stable bottom. Other underlying factors, such as the transport or assimilation of particulate material in the water column, may be being definitive for the biota identified below the tanks.
Fish feeding and stocking density at the marine farm in the Bananal Cove are not evaluated in this study but should not be discarded since the production of waste is essential for the balance between the activity and the environment where it is installed.
Despite the need for future research, the results of this study have positive implications for the continuity of activity at the site, not showing negative effects on the background environment due to the entry of organic matter. Considering the results presented in this study and in previous studies, it is possible to consider that the marine farm on site is environmentally sustainable and the activity is possibly not causing impacts associated with the residues produced by the fish in the benthic community of unconsolidated substrate.
The authors would like to thank the Faculty of Oceanography of the University of the State of Rio de Janeiro for providing the equipment and laboratories to carry out the study.
The authors thank the Pousada Nautilus for granting access to the fish farm in the cove of the Bananal.
The present work was carried out with the support of the Coordination for the Improvement of Higher Education Personnel - Brazil (CAPES) - Financing Code 001.
The authors do not have any conflicts of interest.
None.

©2022 de, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.