Journal of
eISSN: 2378-3184


Research Article Volume 11 Issue 1
ICAR- Central Inland Fisheries Research Institute, India
Correspondence: Basanta Kumar Das, ICAR- Central Inland Fisheries Research Institute, Barrackpore, West Bengal, India
Received: January 20, 2022 | Published: March 24, 2022
Citation: Chakraborty H, Manna RK, Mandal S, et al. Assessing growth performance and survival of wild shad Tenualosa ilisha fry in two earthen ponds with different feeding regime: A new insight for conservation. J Aquac Mar Biol. 2022;11(1):9-15. DOI: 10.15406/jamb.2022.11.00330
The present study describes the rearing and growth performance of Hilsa (Tenualosa ilisha) fry in experimental trial performed in earthen pond ecosystem located adjacent to river Rupnarayan for developing in-situ rearing protocol of fry collected from the wild sources. The seed was collected during May, 2020 from the river and stocked in two different rearing ponds. One of the ponds was provided with artificial feed and another fed with chicken offal to raise the production of zooplankton. Routine monitoring of water and soil quality parameters as well as other biotic communities like plankton was performed to understand the dynamics of ecological conditions in relation to changes in length and weight. The results of PCA between sediment quality and specific growth of fish indicated the strong positive loading of available nitrogen, available phosphate and free calcium carbonate, while moderate positive loading was observed with percentage of silt. Interestingly, it is observed that survivability rate of fish was approximately 80% in both the ponds. The results indicated natural growth fish was considerably well in both the experimental ponds. However, higher growth of juveniles were observed in the pond 2 with higher phytoplankton density (9120cells/L) than pond 1 having lower phytoplankton density (920cells/L) at the end of the experiments. The water quality parameters were comparable in both the experimental ponds. Based on this study it is evident that plankton (especially zooplankton) has induced better growth in pond 2 and possible incorporation of live feed along with formulated feed might trigger better growth of the stocked Hilsa. The present study provides new insight in developing field based approaches for successful rearing and conservation of threatened Hilsa in Indian waters.
Keywords: conservation, ecology, growth, Hilsa, PCA, pond
Indian shad Hilsa (Tenualosa ilisha) has long been considered as a fluvial anadromous fish with feeding grounds in the sea and spawning grounds along the stretches of the lower and middle reaches of big as well as small rivers of India. The Hooghly-estuarine system, on the Indian coast of Bay of Bengal, is one of the largest and most productive estuaries in the country.1–3 Occurrence of juveniles offers evidence of a likely spawning season and spawning ground.4 Up to 16cm size range are considered as juveniles as they are caught in the fixed bag net as well as small mesh sized gill net. The availability of Hilsa juveniles in a long stretch of the Hooghly estuary indicates that the stocks still prefer to spawn in this region.5–9 The fish as tremendous fishery importance and is distributed in Asian countries including India, Bangladesh, Nepal, Sri Lanka, Pakistan, China, and United Arab Emirates and also in Myanmar, Iraq, Iran, Malaysia, Oman, Kuwait, Qatar, Saudi Arabia, Thailand and Viet Nam. They feed on plankton mainly by filtering but apparently also by grabbing on muddy bottom and breeds in rivers during the south west monsoon. The fish is socio-economically, culturally and religiously important fish for the people in West Bengal, India and due to its high commercial and conservation value it has been designated as a State Fish of West Bengal, India.10 A large part of the local inhabitants in and around the southern zones of West Bengal depends on the production of Hilsa in the estuarine water. However, it has been reported recently that the average dimension of individuals of Hilsa are on a decline than those in Bangladesh waters.11 The migration pattern of Hilsa population is a major concern in fishery research to identify most beneficial fishing zones and proper maintenance of the state of conservation for further exploitation at the commercial level.
In Bangladesh, Hilsa it is declared as a National Fish. The reports show that though in Bangladesh it is abundant in the Rivers of Meghna River, Padma River, Rupsha River, Sibsha River, Biskhali River, Tetulia River, Arial Kha River, Galachipa Rirver, Pyra River and a small number of other rivers in the coastal area.12 However, in India a drastic decline of wild population wild population has been reported due to several anthropogenic factors like over exploitation, habitat destruction, pollution, construction of dam. Therefore, there is urgent need to develop the rearing protocol under different feeding and environmental regime and develop the field based grow out of this valuable species. In India, Hilsa, contribute nearly 19% of total fish landing in the Hooghly-Matla estuarine system. They are enriched with omega-3 fatty acid which is good for controlling cholesterol and insulin level. The fish contains proteins and other compounds, lipids and very low level of carbohydrates. Narejo et al.13 reported that the diet of Hilsa of ring dam (up streams) of river Indus was mainly composed of three groups of phytoplankton, Bacillariophceae (93%), other plant materials (3.04%) and debris (3.93%).
The review of the literature shows that artificial breeding of Hilsa shad was attempted by stripping of the species collected from Sirsha almost 50km. downstream of Allahabad.14 The same was reared under the river pools and nursery pond with alkaline conditions with a pH of 7.4-7.6. In another study,15 reported the nursery rearing of the pond. In addition to that, Bhanot & De16 used naturally collected Hilsa (25-60mm size) and stocked in a freshwater pond. Sharma17 also stocked 100-400gm size of Hilsa on freshwater pond and Panickar et al.18 stocked artificially bred seed of 20-25mm in Ballavsagar reservoir. Artificially bred seed of Hilsa was raised in a cemented pond for 47 days.19 Bangladesh also attempted for Hilsa aquaculture during 1988, under International Development Research Centre (Canada) trials carried out at Chandpur riverine station.20 A recent initiative of different research groups to successfully rear and grow Hilsa in a confined environment is still in a rudimentary stage20,21 with poor success in nursery management22 and mass mortality mainly attributed to poor water condition in pond and absence of suitable food.2,3 A comprehensive review on the breeding and rearing of Hilsa T. ilisha has been made by Sahoo et al.23 and identified the gaps towards the development of aquaculture practice that needs to be developed through regional cooperation between India, Bangladesh and Myanmar. Looking into the various importance of fish and its slow growing nature, the present study was undertaken to determine the difference of growth in two experimental ponds, the role of plankton diversity and abundance and establish a relation to water quality parameters with the aim of contributing to the knowledge of Hilsa culture as well as conservation of wild stock.
The experimental details
The experimental culture ponds are located in Kolaghat, West Bengal, India located adjacent to Rupnarayan River, which is a tributary of the river Ganges. The site along with two cultured ponds is depicted in Figure 1. The fry was stocked in two different ponds at Kolaghat, West Bengal, and filled with water of the Rupnarayan River. The sampling is conducted over the time period of 8 months from May to December, 2019. The two culture ponds are named as Pond 1 (0.54 acre) and Pond 2 (0.44acre). The river water is the main source of water for the experimental culture ponds.
Feeding schedule
Two ponds were stocked with 1000 numbers each with Hilsa fry with an average 5.2gm weight collected from the river Rupnarayan. Pond 1 was considered as control whereas pond 2 was applied 20kg. of boiled chicken offal per month to develop the natural fish food organism. As the juveniles were collected from the natural source and the community pond nearby to the river were selected for the growth study and its domestication purpose, it was difficult to select more replicates for the experiment purpose. The fish was fed with commercial floating diets of crude protein (24-28%) and 4 percent fat in a graded manner from 3-4mm size, twice a daily at 4% of fish body weight.
Plankton sampling and analysis
The plankton from both the pond as monthly intervals using plankton net made of bolting silk cloth (40μm mess size) by filtering 50lits of subsurface water and fixed in 4% formalin. Further diversity of the plankton was analysed using a phase contrast light microscope and expressed as unit/lit phytoplankton and no /lit for zooplankton.
Water and sediment and chlorophyll characteristics
The surface water samples were collected from both ponds once in a month from May to December. The physico-chemical parameters are temperature, depth, pH, salinity, specific conductivity, turbidity, dissolved oxygen, total alkalinity, carbonate, bicarbonate, total suspended solids, phosphate, silicate, sulphate, total phosphate, total hardness, total solids, total dissolved solids were measured following the method of APHA 2017. Transparency of water is measured by using Secchi discs at two depths (disappearing, reappearing) using a black and white standard colour coded disc. The air and water temperature are measured by using centigrade mercury-in-glass thermometer of range 10-110°C and the results are expressed in degrees Celsius (°C). The hydrogen ion concentration (pH) is determined by the manual method in the field. The dissolved oxygen is measured by Winkler Method. Water is collected for chlorophyll in a sample bottle for laboratory analysis. In addition to the above, soil samples was collected from the bottom of the pond once in a three month during May to December. The major soil parameters considered were pH, specific conductivity, available nitrogen, available phosphate, organic carbon, CaCO3, sand, silt, clay etc. The chlorophyll of the two ponds was measured by following the method of APHA 2017.
Growth parameter
The growth of the Hilsa juveniles was measured on monthly intervals for 10 individuals randomly collected from each pond using drag net (70mm) and the average growth was calculated. As the fish was fast moving, difficult to handle and die immediately, so 10 fish were selected for a single point of time for calculating specific growth and weight gain efficiency. The specific growth rate (SGR) was calculated monthly for a period of 244 days. The SGR refers to a percentage increase in body dimensions per time and the results are given in percentage increase per day.25 The formula for calculating the SGR is as follows:
Weight gain = Fw-Iw
Weight gain (%) = 100 × (FW - IW)/Iw
Specific growth rate (SGR; % d-1) = 100 × [Ln(FW) – Ln(IW)]/d
Iw was initial fish weight (g),
d was duration of the fish rearing (30 days here for monitoring month wise specific growth rate).
Statistical analysis
For water quality and soil parameters and their relationship with growth parameters, the PCA was employed using PAST 4.02 software. The SPSS 22 was used to determine the correlation among the different parameters. Further, 2 tailed t-tests was performed for the chlorophyll study, the correlation was done with the water quality of pond 1 and pond 2 to find out its relationship influencing each other.
The physicochemical parameters of the pond's water are enumerated in Table 1. In the present study, turbidity, dissolved oxygen, total alkalinity, carbonate, bicarbonate, total suspended solids, phosphate, silicate, sulphate, total phosphate, total hardness were varied between the two ponds whereas other parameters e.g. temperature, depth, pH, salinity, specific conductivity, total solids, total dissolved solid did not vary much (Table 1). In pond no.1 the DO showed highest in November but in pond2 it was in August. In both the ponds, the Total suspended solid was maximum in September. Total alkalinity was recorded highest during May and lowest during September in pond 1 but in pond 2 highest in July and September and lowest in August. The maximum sulphate was recorded in the month of July in both the pond but minimum in September and August. Total hardness was highest in May month in both the pond and lowest in September and August. Both table 1 and 2 data are represented as mean±SE and maximum to minimum of physicochemical parameters of the two experimental ponds.
Pond 1 |
Pond 2 |
|||
Mean ±S.E |
Range |
Mean ±S.E |
Range |
|
Air temperature (°C) |
30.63±2.60 |
16.3-38.0 |
30.81±2.59 |
16.3-38.5 |
Water temperature (°C) |
29.7±2.03 |
19.6-35.3 |
29.58±1.96 |
19.3-35 |
Depth (cm) |
1.4±0.14 |
0.9-1.9 |
1.38±0.09 |
0.89-1.65 |
Transparency (cm) |
36.12±3.44 |
28-48 |
37.62±3.24 |
31-57 |
Turbidity (NTU) |
26.5±2.56 |
16.4-36.9 |
38.75±6.11 |
17.1-65.2 |
Specific Conductivity (mS/cm) |
1±0.15 |
0.63-1.5 |
1.21±0.20 |
0.9-1.7 |
pH |
8±0.13 |
7.4-8.4 |
7.91±0.17 |
7.2-8.4 |
Dissolved Oxygen (mg/l) |
7.4±0.18 |
6.8-7.6 |
6.41±0.42 |
5.2-8.2 |
Total Alkalinity (ppm) |
113.7±9.91 |
82-158 |
124.75±4.95 |
94-138 |
Carbonate (ppm) |
18.37±4.66 |
0-32 |
14±4.12 |
0-24 |
Bi carbonate (ppm) |
94.5±6.89 |
66-126 |
110.87±7.62 |
70-138 |
Chlorinity (ppt) |
0.22±0.04 |
0.09-0.4 |
0.27±0.05 |
0.49-0.12 |
Salinity (ppt) |
0.42±0.08 |
0.18-0.76 |
0.50±0.10 |
0.9-0.2 |
Salinity by Specific Conductivity (ppt) |
0.30±0.1 |
0.1-0.7 |
0.36±0.12 |
0-0.1 |
Total solid (g/l) |
0.81±0.11 |
0.3-1.1 |
0.87±0.13 |
0.84-1.43 |
Total Dissolved solid (g/l) |
0.74±0.11 |
0.3-1.1 |
0.79±0.14 |
0.54-1.4 |
Total suspended solid (g/l) |
0.05±0.02 |
0.02-0.1 |
0.62±0.03 |
0.009-0.2 |
Nitrate (ppm) |
0.02±0.009 |
0.008-0.02 |
0.02±0.007 |
0.009-0.01 |
Total Nitrate (ppm) |
0.14±0.06 |
0.09-0.1 |
0.19±0.06 |
0.06-0.18 |
Phosphate (ppm) |
0.04±0.005 |
0.06-0.01 |
0.16±0.103 |
0.06-0.1 |
SiO4-Si (ppm) |
4.8±0.63 |
2.95-7.8 |
5.4±0.66 |
2.6-8.5 |
Sulphate (ppm) |
23.81±14.31 |
0.09-109.48 |
16.57±9.33 |
0.08-71.55 |
Total Phosphate (ppm) |
0.5±0.20 |
0.048-1.4 |
0.37±0.11 |
0.05-0.1 |
Total Hardness (ppm) |
185.75±27.40 |
124-300 |
230.37±30.37 |
152-380 |
CA ++ (ppm) |
32.6±5.13 |
22.4-64.12 |
32.03±3.06 |
19.23-48.09 |
MG++ (ppm) |
25.21±4.86 |
12.9-47.4 |
32.64±6.11 |
20.12-62.34 |
Table 1 Physico-chemical parameters of the two ponds from May to December
Principal Component analysis done to understand which sediment factors affects the specific growth of the Hilsa in pond. The colour coding are showing different month, plus sign shows pond 1 and dot sign shows pond 2. For the analysis screen plot is created for >1 Eigen value. For the 3 PC’s were extracted given in Table 2 in which for first 3 PC’s the cumulative variance % was 96.411 %. The 1st, 2nd and 3rd PC’S contributed with 44.001%, 35.901 % and 16.509%. The loading of PC1 indicated the strong positive loading of available nitrogen, available phosphate and free calcium carbonate. While moderate positive loading was observed with Silt %. Different loading value of extracted PC’s are given in Table 3. In the 1st PC, the strong negative loading was observed with clay %, while moderate negative loading was observed with specific growth %. The 2nd PC has strong positive loading with organic carbon and specific growth %. A moderate positive loading was observed with conductivity and available phosphorus. In the PC strong negative loading for pH, while moderate negative loading was observed with free calcium carbonate. The 3rd PC has moderate positive loading of sand and silt %. The analysis indicated also that specific growth % showed strongly positive influence for organic carbon while moderate effect of conductivity and available Phosphorus. In this study, the variation of chlorophyll a and b in pond 1 and pond 2 were significantly different (p<0.05). Similarly, total chlorophyll has a similar trend to chlorophyll a and b. However, chlorophyll c do not varied significantly in both rearing ponds.
PC |
Eigenvalue |
% variance |
1 |
4.40011 |
44.001 |
2 |
3.59013 |
35.901 |
3 |
1.65091 |
16.509 |
Table 2 Eigen value and variance %
PC 1 |
PC 2 |
PC 3 |
|
pH |
-0.25002 |
-0.94577 |
0.12889 |
COND |
0.36218 |
0.5849 |
0.69422 |
AN |
0.8918 |
-0.31445 |
0.19536 |
AP |
0.79969 |
0.54361 |
0.16033 |
OC |
0.42482 |
0.85033 |
0.29595 |
FCACO3 |
0.78668 |
-0.5491 |
0.13 |
SAND |
-0.56266 |
-0.3433 |
0.74182 |
SILT |
0.73973 |
0.18588 |
-0.64408 |
CLAY |
-0.84757 |
0.46185 |
-0.10393 |
SG% |
-0.62459 |
0.75424 |
-0.08985 |
Table 3 Loading value of different extracted PC’s
Pattern of fish growth
In the present study, the highest mean length and weight were found in pond 2 (59.76±13.65cm) and (78.52±20.79cm) more than pond 1 (25.16±6.09cm) and (38.11±11.04cm) as shown in Figure 4. The difference in weight gain % of the last month in pond 1 was 15.85% and it was higher 17.60% in Pond 2. The SGR attained at the end of the study (after 244days), the highest SGR of pond 1 and pond 2 in June was 2.91% per day and 5.23% per day indicating a promising increment rate. The overall growth attained after 244days and the specific growth rate of pond 1 and 2 per day was 0.388% and 1.550%, respectively.
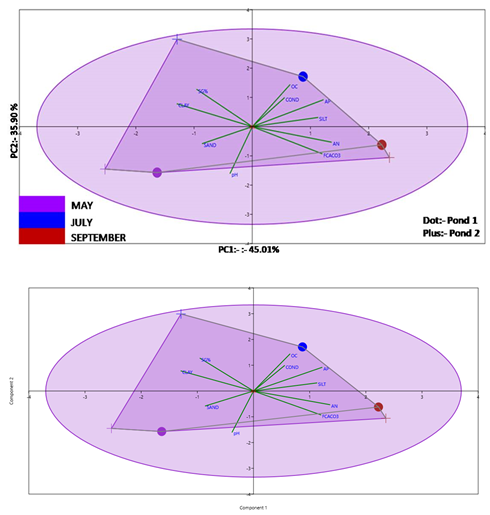
Figure 2 PCA bi-plot of different sediment parameters affecting specific growth rate of Hilsa for both the ponds.
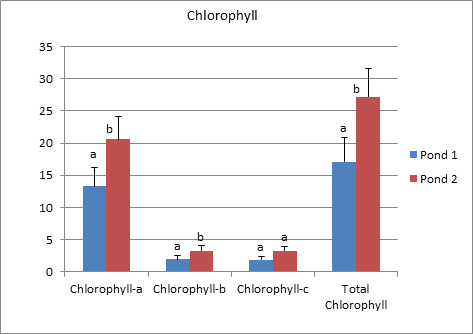
Figure 3 Pattern of chlorophyll in the both ponds during the study period(chlorophyll-a, chlorophyll-b, chlorophyll-c, total chlorophyll). Data represents (mean±SE), Mean bearing common superscript are not significant to each other.
The physicochemical parameters:
Principal Component Analysis (PCA) is the effective statistical tool used for analysing major influencing factors responsible for pond production. It was performed by taking 27 important water quality parameters i.e. Air temp (AT), Water temperature (WT), Depth, Transparency (Trans), Turbidity (TUR), Sp. Conductivity(Sp.CON), pH, DO, Total Alkalinity (TALK), Carbonate, Bicarbonate, Free CO2, Chlorinity (chlo), Salinity(SAL), TS, TDS, TSS, Total N, Silicate, Total P, Total Hardness, Calcium, and Magnesium. For both, ponds, a PCA bi-plot was created to know the differences. In the PCA bi-plot, it has been found that the cumulative variance percentage for PC I and PC II was 62.82 % and among the PCI, PC II, and PC III the major driving factors are water temp, Chlorinity, Total alkalinity, salinity, sp. cond, TS, TDS, and Magnesium. In the analysis, the major factor (Figure 5) influencing specific growth % were water temperature, air temperature, pH, carbonate, and total nitrogen. In pond 2, the maximum of growth was observed in the month of June, while the maximum sp. growth % in pond 2 was in July (Table 4). The colour coding is shown different months and plus sign shows pond 1 and dot sign shows pond 2.
PC |
Eigenvalue |
% variance |
1.00 |
13.04 |
48.29 |
2.00 |
3.92 |
14.53 |
3.00 |
2.43 |
8.99 |
4.00 |
1.81 |
6.69 |
5.00 |
1.63 |
6.04 |
6.00 |
1.18 |
4.38 |
Table 4 Eigenvalue and variance %
Comparison of plankton abundance between two ponds
The phytoplankton and the zooplankton density were monitored in two ponds throughout the study period (8months). The phytoplankton density was higher at pond 2 as compared to pond 1. The variation of the phytoplankton ranges from 520 to 920 number of cells per litre in pond 1. Whereas, in pond 2, this variation was 42 to 9120 number of cells per litre. The zooplankton density was higher in pond 1 as compared to pond 2. The variation was found to be 1540 numbers of individual per litre from the first month to 1865 individual per litre in the 8th of the study period. Whereas in pond it varied from 2,44 to 780 individual per litre. The dominant group of phytoplankton was Aulacoseira, Pediastrum, Actinastrum, Spirulina, Anabaena, Phormidium, Microsystis, Merismopedia, Oscilotoria, Chrococcusand the zooplankton were Keratella, Cyclops, Brachionus, Filinia, Rotifera.
A very few attempts were made earlier to experimentally rear wild collected fry in ponds under different feeding and environmental regime although few reports are available on larval rearing of captive breed Hilsa with low to moderate success.16,17 The present study demonstrated the growth Hilsa fry (T. ilisha)in experimental ponds and considerable survivability of fry was observed. The synthesis of earlier reports available indicates a lack of systematic documentation and standards with reference to growth, monitoring of water quality, different feeding trials, role and relationship of water quality and ecological perspectives. Malhotra et al.14 reported artificial breeding of Hilsa was by stripping off the species in downstream of Allahabad and reared in the river pools and nursery pond. The hatchling of 2.5-3mm size attained up to 10mm during 20 days rearing period. In another study15 reported the nursery rearing of the Hilsain pond with a pre stocking management nursery pond @3.5-5million/ha and recorded survival up to 80-90% with a hatchling of 4-5mm size. Bhanot and De16 used naturally collected Hilsa (25-60mm size) and stocked in a freshwater pond in a 1 ha size. They reported attainment of the harvestable size of 125gm/300gm/425gm at the end of 1year/2year/8year 8 months, respectively. However, the survivability and other records on the influence of water quality and feeding types with replication are not available; Sharma17 also stocked 100-400gm size of Hilsa in freshwater pond and harvested 300-800gm within 1yr 6month. Similarly, Panickar et al.18 stocked artificially bred seed of 20-25mm in Vallabhsagar reservoir for 1 year 5 months and harvested size range between 500-600gm. Artificially bred seed of Hilsa was raised in a cemented pond for 47 days5 and natural collection seed up 20mm size was cultured for 5months in a freshwater tank within the harvested size of 121mm.25 The reports from Bangladesh indicate low to moderate success in breeding and larval rearing of Hilsa under different programs.19 A recent initiative taken by another author to grow Hilsa in a confined environment but still the progress is in a rudimentary stage.19 More recently, Chattopadhyay et al.21 reported larval rearing of Hilsa (4 days old, 4.76±0.06 mm) stocked in fibreglass-reinforced plastic tanks reared for 46 days. The larvae were supplied with Chlorella vulgaris, Brachionus calyciflorus, mixed phytoplankton and mixed zooplankton during 4–50, 6–25, 8–50 and 26–50 days of their age respectively. They reported good survival of fry was through feeding initially with Chlorella followed by Brachionus sp., mixed phytoplankton and zooplankton.
The studies on the habitat requirement and ecological characteristics of the pond reared Hilsa juvenile is very limited. In the present study, the water quality parameters were comparable in both experimental ponds 1 and 2. In the present study the PCA indicated that the major factor influencing the specific growth % were water temperature, Air temperature, pH, carbonate, and total nitrogen. In pond 1, maximum growths were observed in the month of June, while the maximum sp. growth % in pond 2 was in July. Limited reports are available on the growth performance and role of ecological variables. The studies of Hossain et al.26 reported assemblage of marine brackish-freshwater ecosystems with favourable ecological parameters and rainfall patterns are very much important for supporting juvenile Hilsa in the study area. The most suitable zones for juvenile Hilsa were correlated to favourable water temperature (23–27°C), dissolved oxygen (>5mg/L), pH (6–8), salinity (<0.5‰), turbidity (<100mg/L), current (0.15–0.20m/s), depth (<10m) and rainfall (200–225mm), although some of the parameters are life stage dependent.26,27 This critical assessment on the hydrobiological, climatic, chemical and biological profile of the environment of the Hilsa rearing pond habitat provides location specific information which is essential to understand the preference of different life stages for fisheries management.
The present study has provided a clear understanding of the growth increment and feed preference of Hilsa fry at different life stages, with insights into ecological conditions of the rearing system. The survival rate of fish in the rearing pond was encouraging (80%). The 8 months study indicated that plankton (especially zooplankton) has induced better growth in pond 2 and possible incorporation of live feed along with formulated feed might trigger better growth of the stocked Hilsa. The report on the feeding trials using a combination of natural and formulated feed is not available although considerable studies are available on the feeding habit of fish from Bangladesh and India.19 The review of the literature shows that Hilsa preferred phytoplankton over zooplankton though the opinion differs with varied results employing different methods. Among phytoplankton, Bacillariophyceae (diatoms) were the most preferred Hilsa food organisms, especially in the early stages of the life cycle.27 Jones & Sujansingani28 stated that Hilsa was essentially a plankton feeder and did not exhibit any selection in feeding while Narejo et al.12 reported that Hilsa avoided zooplankton and showed a strong preference for some genera of phytoplankton, such as Bacillariophyta, Chlorophyta and Cyanophyta. Al-Nasiri & Mukhtar29 while working on Hilsa from Asharcanal, Basrah (Shatt al –Arab River), Iraq, concluded that the main food of this species is zooplankton (mainly copepods- Cyclops) and phytoplankton such as dinoflagellates and diatoms.
The present study demonstrated the growth performance and hydrological preference of Hilsa in experimental ponds and considerable survivability of fry was observed as compared to previous studies reported. The study provides new insight in developing field based approaches for the rearing of Hilsa fry with considerable success in the growth and survival of fish in two different rearing systems and established the role of plankton and hydrobiology in triggering growth performance. The research on conservation and enhancement of Hilsa fisheries requires a lot of dedicated approaches to thrash out problems and issues. Developing standard fry and juvenile rearing protocol of natural gene pool through establishing in-situ conservation approach and adopting adequate measures for stock enhancement are important for promoting conservation based culture practices. Public awareness of the necessity of conservation and propagation needs to be created through mass media. Ban on indiscriminate and destructive fishing practices to protect the young and juvenile Hilsa. The existing laws and regulations should be enforced properly to protect this high value fish. The study suggests more field based research at different geographical locations to upscale the in-situ rearing technique and sustainable fisheries in the Ganges basin.
The authors acknowledged to the director, ICAR-CIFRI for providing facility to carry out the research work.
The experiment was designed by BK D. Field data were collected and analysed by HC, RKM, AKS, SM. Statistical analysis were analysed by HC. Data interpretation by BKD, AKS, UKS. Manuscript is prepared and editing by all the authors.
The availability experimental data generated or analysed in the present study are available from the corresponding author on request.
The experiment conducted in the present study has been passed through institute ethical committee and the committee approved to carry out of experiment sampling of fish and its handling were performed as per the instruction laid by the ethical committee./p>
The author declared that there is no conflicts of interest.

©2022 Chakraborty, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.