Journal of
eISSN: 2378-3184


Research Article Volume 7 Issue 4
1Department of Marine Science Mawlamyine University Myanmar
2Marine Science Association Myanmar MSAM Yangon Myanmar
Correspondence: Jar San Assistant Lecturer Department of Marine Science Mawlamyine University Myanmar
Received: June 20, 2018 | Published: July 16, 2018
Citation: San J, Soe-Htun U. Spore germination on Polysiphonia subtilissimaMontagne from Setse and Kyaikkhami coastal areas, Thanlwin river mouth, Myanmar. J Aquac Mar Biol. 2018;3(4):194?197. DOI: 10.15406/jamb.2018.07.00208
Polysiphonia subtilissima Montagne collected from Setse and Kyaikkhami coastal areas was carried out. The carpospores of P. subtilissima germinated at 25 under the photoperiod of 8L:16D and 30˚C under the photoperiod of 16L:8D, showing the early stages of cell divisions and development of thalli in culture. The carpospore germination pattern of P. subtilissima Montage of laboratory experiment was briefly discussed.
Keywords: carpospores, kyaikkhami, laboratory culture, polysiphonia subtilissima, Setse, germination
The genus Polysiphonia is widely distributed in most temperate and tropical waters of the world.1Recently, Guiry and Guiry2 recorded 119 species of Polysiphonia reported from various regions of world oceans.
Kyaw Soe & Kyi Win3 had also reported 9 species of Polysiphonia. However, Kyaw Soe & Kyi Win3 and Soe-Htun4,5 gave no specific description on Polysiphonia spp. due to lack of specimens mentioned in the previous reports, in the Department of Marine Science. Recently, Soe-Htun et al.6,7 listed two species of Polysiphonia viz., P. subtilissima Montagne and P. sp, Soe-Htun et al.,8 recorded the only species of P. subtilissima Montagne along Gwa coastal areas. Sein Moh Moh Khaing9, Jar San,10 ZayarAung,11 Myo Min Tun,12 Thet Htwe Aung13 and EiEi Hlaing14 described the habitat of P. subtilissima Montagne.
The carposporangia release the carpospores to the marine currents and tides. They settle down on a surface and germinate by mitosis and grow into the diploid tetrasporophyte. The tetrasporangium has four (tetra) tetraspores packed into each tetrasporangium. These meiotic products are in a tetrahedral arrangement. Each spore has a circular contact area with the other three spores in the tetrahedron, and the spore will have a tri-radiate ridge marking in this circular contact area. The purpose of this study is to know the early developmental stages in carposporegermlings of P. subtilissima in laboratory cultures as well as in the field.
The plants of Polysiphonia subtilissima Montagne were collected from the mangrove swamp and rocky platform in the intertidal zone at Setse (Lat. 15°52'N, Long. 97°35'E) and Kyaikkhami (Lat. 16°05'N, Long. 97º34' E) coastal areas, Thanbyuzayat Township, Mon State (Figure 1) during the period from June 2014 to September 2015. The materials were brought in ice box to the laboratory at Mawlamyine University for the observation.
The cultured apparatus such as Petri dishes (7.5x1.8cm) and (4x2cm), glass slides, blades, brushes, glass bowls, cover slips, pipette, pointers, cover slips (1.7x1.7cm) and forceps were washed with tap water and then sterilized again with boiling water. Sterilized seawater was adjusted to get salinity by refractometer. And then Iwasaki’s SWII media was prepared for both spore liberation and germination. Germanium dioxide (GeO2) was employed for elimination of diatoms throughout this study.
In the laboratory, the fresh and healthy carposporophyte plants were randomly selected and dried with tissue paper and were kept under the dark condition for overnight. In the next morning, both reproductive fragments were placed on cover slips in each Petri dishes (7.5x1.8cm) in 30ml and (4x2cm) in 20ml of prepared culture medium. These Petri dishes were placed under at 25˚C under the photoperiod of 8L:16D and at 30˚C under the photoperiod of 16L:8D, using cooled and ventilated incubators (Gallenkamp Cat No. IMF 781-7, volt 220/240, MHz 50). Aeration was not provided in all experiments.
Observations on liberation of spores were carried out hourly with intervals of 1 or 2hrs over a period of 24hrs to 48hrs of collection in the laboratory. Carpospores settled on the cover slips were transferred to each Petri dish containing prepared culture medium. The numbers of cell divisions were recorded and sizes of spores and germlings were measured under the compound microscope using ocular meter in 3days interval. The developmental stages of carpospores germlings were photographed by Sony DSC-W 330 digital camera, processing with Adobe Photoshop CS3. The culture medium was changed at 3days interval. Culture studies were repeated several times. This study followed the classification system of used by Guiry and Guiry.2
A classification system of the Polysiphonia subtilissima Montage
Phylum: Rhodophyta
Class: Florideophyceae
Order: Ceramiales
Family: Rhodomelaceae
Genus: Polysiphonia Greville
Species: Polysiphonia subtilissima Montagne
Stages in the development of carpospores germination in Polysiphonia subtilissima Montagne
Germination of carpospores and further development of the germlings could be studied by using a broad range of salinities 30‰ at 25°C under 8L:16D photoperiod. The carposporelings of P. subtilissima liberated at all salinities though the sporelings did not germinate in lower salinities 25‰ and higher salinity of 30‰. The germling of P. subtilissima developed into polysiphonous structures.
Liberated carpospores were globular and deep red in color; they have thin transparent walls and vary from 50µm to 70µm in diameter (Figure 2) (Figure 3) (Figure 13). The liberated carpospores were clavated and gradually transformed into globular within 5minutes. Average mean lengths of carpospores germlings of P. subtilissima during after fifteen days culture period were observed in (Table 1) (Figure 23).
First, isolated carpospores attached to the substratum within twelve hours and three unequal transverse walls are formed 80µm in diameter. This cell division takes place within 12 to 24hours (Figures 4) (Figure 14). After 24 to 36 hours, the carposporelings continued it division to from four-celled stage, have 100µm length and 80µm broad (Figures 5) (Figures 15).
After three days initiating, the basal portion of sporeling became the rhizoid shoot (Figure 6) (Figure 16). The rhizoidal pole was conical in shaped, continually lengthened and reached 15-30µm in length.
After 5days, germling continued four celled divisions and the rhizoid cells divided into 1-2cells reaching a length of 120-130µm in length and 50µm in width (Figure 7) (Figure 17). And then, (Figure 8) (Figure 18) shows each segment of the main axis was composed five cells and the rhizoid cell divided into 3 - 4 cells, length varied from 350-400µm in length by 6 days development.
After 7days germling, about 6-8cells in length with a longitudinal cell showing formation of the rhizoidal cells are found in five cells. The main axis was recurved and an expanded disc-like rhizoid, have 400-600µm in length 50-70µm in width (Figure 9s) (Figure 19). Within 7-10days, the cells number was 8-12cells that axial cell at a position of 2-3 segments behind the apical cell were divided to form the lateral pericentral cells and the first lateral initials form the newly cutoff pericentral cell, 400-600µm in length of thallus and 400-500µm in length of rhizoid (Figure 10) (Figure 20).
Germlings appeared the number of several cells with developed rhizoids after 12days (Figure 11) (Figure 21). After 15days inoculation, the pericentral cell cut off and formed as polysiphonous structure in the young filamentous sprout. The germling increased in length and growing, measuring a total length of up to 1200µm. The color of the germling was normal reddish brown (Figure 12) (Figure 22).
In the laboratory studies, the effects of temperature on spore germlings of Polysiphonia were investigated at 25°C and 30ºC. The growth of carposporelings was higher at 25ºC, but did not distinctly different occur at 30ºC. Kudo and Masuda15 observed the optimum salinity for the growth of carpospores germling of P. japonica and P. akkeshiensis were found under 10 - 20°C and photoregimes (16L:8D and 8L:16D).
Carpospores discharge of P. subtilissima was observed within 1-8hrs whereas Kudo & Masuda15 reported the spore discharge of P. japonica Harvey and P. akkeshiensis Segi were observed within 24hours. Kudo & Masuda15 described, liberated carpospores were globular, deep red in color and they average 60.3µm (range 57.5-65.0µm) in diameter. In P. akkeshiensis liberated carpospores deep red in color and average 57.6µm (range 52.5-65.0µm) in diameter.
The species of P. japonica and P. akkeshiensis isolated carpospores soon attached to the substrate and grew into bipolar sporelings of 6-7 segments, which one day after inoculation, had differentiated into a colorless rhizoid and a pigmented main axis. These two species of germlings lateral initial were formed from each segment in a spiral line running in a counter clockwise direction toward the apex of the main axis as development of the main axis proceeded. The first lateral initial grew usually into a pseudo dischotomously divided vegetative trichoblast. However, the species of P. japonica and P. akkeshiensis formed all the ordinary branches grew indeterminately as did the main axis forming vegetative trichoblasts and ordinary branches of the second order.
According to the (Table 1), the spore germination grew quickly and the germlings gradually died after 20days. In the present study, the carpospores germination of growth rate of the P. subtilissima Montagne is slower than the growth of the carpospores germling of P. japonica and P. akkeshiensis Kudo & Masuda.15 In addition, carpospores form germlings but tetraspores were not germlings in the laboratory experiments.
The local distribution ranges of P. subtilissima Montagne distributes along the Rakhine Coastal Region: from Sin Phyu Gyaing to Shwe Ya Gyaing; Ayeyawady Coastal Region: no data and; Tanintharyi Coastal Region from High Island to Lampi Island. The species of P. subtilissima Montagne is widely distributed throughout the Atlantic Ocean, the Indian Ocean and the Pacific Ocean in tropical and temperature water. Further studies are still needed to verify and revise the spore germination patterns and life cycle of the genus Polysiphonia Greville from Myanmar.
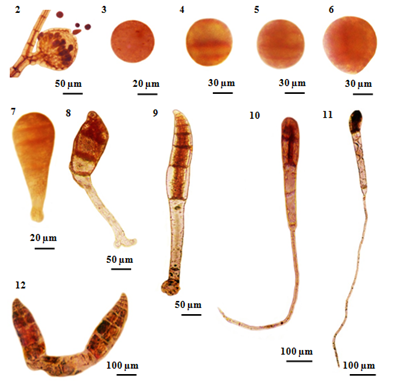
Figure 2 Carpospores and their early development of Polysiphonia subtilissima Montagne. (2) Liberated carpospores from mature cystocarp. (3) Released carpospores. (4)Three unequal- celled stages formed within 12-24 hours. (5) The second segmentation of carpospores germling showing unequal four cells with 24-36 hours. (6) Beginning of germination three days after initiating. (7) Four-celled stage 3-5 days after initiating. (8) The rhizoidal portion of sporeling curved slightly (arrow) after 6-7 days. (9) The germling resulted in 6-8 cells stage and an expanded disc-like rhizoid; the main axis becoming slightly curved after 7 days. (10) After 7-10 days germlings, the number of 8-10 cells, lateral pericentral cells. (11) After 12 days germlings, the number of several cells and rhizoidal cells. (12) After 15 days old carposporelings.
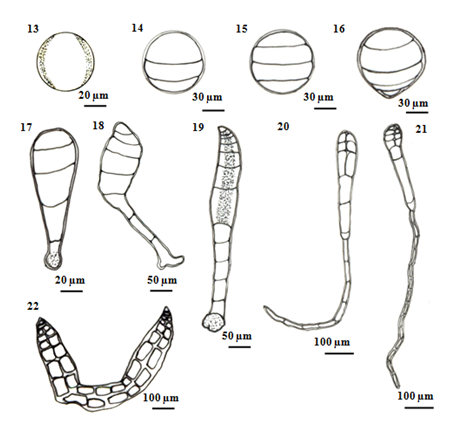
Figure 3 Schematic drawing showing successive stages of carpospores germination in P. subtilissima Montagne. (13) Released carpospores. (14) Three unequal-celled stage formed within 12 - 24 hours. (15) The second segmentation of carpospores germling showing unequal four cells with 24 - 36 hours. (16) Beginning of germination three days after initiating. (17) Four-celled stage 3-5 days after initiating. (18) The rhizoidal portion of sporeling curved slightly after 6-7 days. (19) The germling resulted in 6 - 8 cells stage and an expanded disc-like rhizoid. the main axis becoming slightly curved after 7 days. (20) After 7-10 days germlings, the number of 8-10 cells, lateral pericentral cells. (21) After 12 days germlings, the number of several cells and rhizoidal cells. (22) After 15 days old carposporelings.
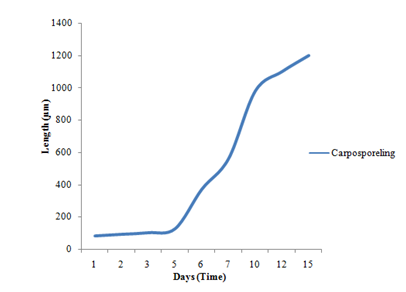
Figure 4 Average means length of carpospores germling of P. subtilissima Montagne during 15 days culture periods.
Days |
Length (µm) |
1 |
80 |
2 |
90 |
3 |
100 |
5 |
125 |
6 |
370 |
7 |
560 |
10 |
980 |
12 |
1100 |
15 |
1200 |
Table 1 Average mean length of carpospores germlings of Polysiphonia subtilissima Montagne after 15 days culture period at temperature of 25ºC under 8L: 16D photoperiod
The plant of Polysiphonia subtilissima abundantly grew along the intertidal region of Setse and Kyaikkhami coastal areas where salinity range was 18-30‰. Field collected plants with cystocarps were selected and cultivated for the early developmental stages of spore germination of P. subtilissima under laboratory condition. In the carpospores germination of P. subtilissima, the pericentral cell cut-off and formed as Polysiphonous structure in the young filaments sprout. The germling increased in length and growing, measuring a total length of up to 1200µm after 15days. The color of the germlings was normal reddish brown in color.
We are thankful to Dr. Aung Myat Kyaw Sein, Acting-Rector, Dr. Mie Mie Sein and Dr. San San Aye, Pro-Rectors of Mawlamyine University, for permission to carry out this research work. We are deeply indebted to Dr. Min-Thein, Chairman, June Pharmaceutical Ltd., General Manager (Retd.), Myanmar Spirulina Factory, Sagaing and Professor (Part-time), Department of Botany, University of Mandalay, for his valuable suggestions and constructive criticism on this study, and literature provided. We would like to thank Dr. San Tha Tun, Professor and Head of Department of Marine Science, Mawlamyine University for providing the departmental facilities. We would like to express our sincere thanks to the staffs of Setse Aquaculture Research Center who kindly help us in many ways during our field trips. Special thanks go to Dr. Soe Pa Pa Kyawand Dr. Mya Kyawt Wai, Lecturers and Dr. Sein Moh Moh Khaing, Assistant Lecturer of Department of Marine Science, Mawlamyine University, for their helpful assistance during this work. The first author, Jar San would like to thank her beloved grandfather Du WaNh kum Zau Gawng, parents U Nhkum Naw Sant and Daw Langwa Zung Lwi, all my siblings and relatives from Kachin Land for their physical, moral and financial which supports made to reach the goal of this work.
Author declares that there is no conflict of interest.

©2018 San, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.