Journal of
eISSN: 2378-3184


Research Article Volume 6 Issue 4
1CSIR-National Institute of Oceanography, India
2Central Marine Fisheries Research Institute, India
Correspondence: Raveendran TV, CSIR-National Institute of Oceanography, Cochin-18, Kerala, India
Received: July 21, 2017 | Published: October 13, 2017
Citation: Karati KK, Vineetha G, Raveendran TV, Muraleedharan KR, Achuthankutty CT (2017) Plankton and the Invisible Barriers in the Tropical Ocean. J Aquac Mar Biol 6(4): 00162 DOI: 10.15406/jamb.2017.06.00162
Diel vertical migration (DVM) is one of the major biological rhythms observed among zooplankton. In tropical environments (TrE), zooplankton is believed to exhibit prominent DVM. Chaetognath, a major vertical migrator among zooplankton in the global ocean, was studied from the Bay of Bengal, an important zone in TrE of Indian Ocean. Here a novel method (abundance-weighted tolerance index) was introduced which was successful in predicting the species, capable of performing DVM. The vertical profiles of physico-chemical variables exhibited a wide variation in upper 1000 m and only two species of the total twenty-five species exhibited significant DVM which opposed the general concept of DVM in TrE. The pronounced variations in physico-chemical variables acted as invisible barriers for zooplankton to perform DVM. The observed restricted DVM will have significant impact on the marine ecological processes including biological pump in the system.
Keywords: Zooplankton, Diel vertical migration, Vertical profiles, Abundance-weighted tolerance index, Marine ecology, Tropical
Diel vertical migration (DVM) is a well-documented behavior among diverse taxa of zooplankton from tropical environments (TrE)1--to higher latitude environments,4-5 and the adaptive significance of this behavior has been explained by a wide array of competing hypothesis.6s The major driving factors of this behavior can be attributed to predator avoidance,7 prevention from light related mortality,8 the bioenergetic advantage,9 and demographic benefits.10 The regulating factors of DVM often differ based on the availability of food, density of predator, the size of an organism,11 and among different life stages.12 We expect that, in TrE, which ever be the driving factor, the organism has to be tolerant to a wide range of the physico-chemical variables to successfully perform DVM as the TrE in the low-latitude regions are characterized by high variation in the vertical profile of physico-chemical variables compared to the middle and higher latitude environments.13-14
Although several methods are being employed to identify the DVM process, like the vertical profile of density values15-16 to acoustic technique,4,17 it is not easy to predict whether any zooplankton group will perform DVM or not. Species-wise abundance-weighted optimum values (AO) and tolerances (AT) for different physico-chemical variables are being used mainly for palaeoecological researches.18 We have modified these equations for the vertical profile of these variables in the marine system. Along with these modified equations, we introduce “abundance-weighted tolerance index (ATI)” to predict the DVM process in TrE. For this purpose, we studied chaetognath, one of the major zooplankton group and active vertical migrator in the global ocean. The abundance-weighted approach is novel in the understanding of the species specific DVM of zooplankton. Our aim was to understand the impact of the wide variation in the vertical profile of the major physico-chemical variables on DVM and to predict the possibility of different species to perform DVM in this environment.
Sampling strategy
Sampling was carried out in the Bay of Bengal during November 2005 to January 2006 as part of the Marine Research on Living Resources program. Stations were distributed along five zonal transects from 11 °N to 19 °N (Figure 1). Diurnal observations were performed at one coastal station and one oceanic station along each transect at 6-hour intervals for 24 hours.
Figure 1 Sampling locations in the Bay of Bengal. The filled squares denote diurnal stations and the circles denote regular stations.
An SBE Seabird 911 plus CTD was used to obtain the temperature and salinity profiles of the water column at each station. For estimation of dissolved oxygen, water samples were collected using a Rosette sampler from standard depths to 1000 m (surface, 10, 20, 30, 50, 75, 100, 150, 200, 300, 500, 750 and 1000m) and analyzed using Winkler’s method.19 Zooplankton was sampled using a multiple plankton net with a mouth area of 0.25m2 and a mesh size of 200 µm (Hydrobios, Germany) from five discrete depth zones, including the mixed layer depth (MLD), the thermocline, the base of the thermocline (BT) to 300 m, 300 - 500 m and 500 - 1000 m. As each net is opened and closed independently, contamination is apt to be negligible.20 The mixed layer depth was determined as the depth where the density difference from the surface was 0.2 kg m-3. The base of thermocline was calculated as the depth where the temperature reached 15 °C. Chaetognaths were sorted from the whole sample or from an aliquot (50%) using a Folsom splitter and counted under a stereomicroscope. The detail species level analysis was carried out following Nair.21 Though the genus Sagitta is sometimes divided into few new genera,22,23 the systematics is based on marginal differences between genera and hence we followed the classical taxonomy24-30 where the genus Sagitta sensulato is used.
Abundance weighted values
We modified the species specific abundance-weighted optimum (AO) and abundance-weighted tolerance (AT) equations of Fritz et al.,18 for making it suitable for the vertical profile of the physico-chemical variables. We introduce here the abundance-weighted tolerance index (ATI),
Where yik is the abundance of taxon k in sample i
xij is the mean value of jthphysico-chemical variable of sample i
AOkj is the abundance-weighted optimum of species k for the jthphysico-chemical parameter
ATkj is the abundance-weighted tolerance of species k for the jthphysico-chemical parameter
ATIkj is the abundance-weighted tolerance index of species k for the jthphysico-chemical parameter.
Statistical Analysis
To check whether a significant variation exists in the abundances of chaetognaths among different sampling depth layers, one-way analysis of variance (ANOVA) was performed. Before the analysis, the D’Agostino and Pearson omnibus normality test was carried out to check their normality in distribution, and based on the result, parametric or non-parametric ANOVA was performed. A paired t-test was carried out between the day and night abundances (loge transformed values) of different chaetognath species at various sampling depths at the diurnal stations, to check the DVM behavior, using Graph Pad Prism (version 5.01). Similar to ANOVA, before the t-test also normality test was done and based on that parametric or non-parametric paired t-test was carried out.
Physico-chemical variables
During the study, sea surface temperature varied between 25.9 and 27.7 °C with an average of 26.8±0.4 °C (Figure 2). In the vertical profile, though just below the surface the temperature was slightly higher (mostly in the northern transects), below that temperature gradually decreased towards depth (Figure 2). The vertical profile of the temperature, based on the mean values of all the sampling stations, showed wide variations in the upper 1000 m (Figure 3). The temperature variation was 20 °C in the 1000 m water column with a difference of 15 °C in the upper 300 m (Figure 3).
The sea surface salinity ranged between 31.6 and 34.1 with an average of 33.1±0.5. Though surface salinity gradually decreased towards the north, the variation between the northernmost (19°N) and southernmost transect (11°N) was only ~1 (Figure 4). The vertical profile of salinity showed a marked gradient in the upper 100 m in the northern part and in the upper 75 m in the southern part. The vertical profile based on the mean values of all the sampling stations also showed the existence of gradual increase of salinity in the upper 100 m with marked gradient and the variation in the deeper water was quite less (Figure 3).
During the study, the surface DO varied from 4.4 to 5.3 ml l-1 with an average of 4.7±0.2 ml l-1 (Figure 5). The vertical profile exhibited a marked gradient in the upper 75 m in the southern part and upper 120 m in the northern part. A thick layer of oxygen minimum zone (<0.5 ml l-1) prevailed below subsurface layer, and it gradually increased towards the north (Figure 5). The mean values of all the sampling stations also clearly exhibit a sharp decrease of DO with depth and the presence of a thick layer of oxygen minimum zone between 100-670 m ().
The MLD varied widely between different sampling locations and ranged between 11-69m (Figure 6a). The average MLD during this season was 41±17m. The MLD showed an increasing trend towards north. In general, the MLD in the coastal region was lower than that observed in the oceanic region. The bottom of thermocline layer also varied widely from 75-225 m with an average of 171±32 m (Figure 6b).
Chaetognatha abundance
The abundance in the upper most layer (MLD) was relatively high [range, 2133 to 78181 in (1000m)-3,] compared to all other layers and the variation in the abundance among the depth layers were statistically significant (P<0.05). In the thermocline layer, chaetognath abundance was relatively high in the northern part (Figure 7). In the layer below, the abundance gradually increased towards the south, and the maximum was observed in an oceanic station along 11°N transect (Figure 7). In the deeper layer, in 300 - 500 m depth also, chaetognath abundance in the southern BoB was relatively high than the northern part. The abundance gradually decreased towards the greater depth with an average of 150±190 in (1000m)3 in 500-1000 m depth (Figure 7).
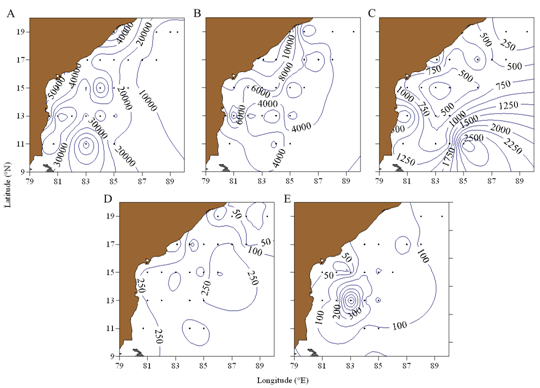
Figure 7 Abundance of chaetognaths [ind(1000 m)-3] along different depths in the Bay of Bengal (a) mixed layer depth, (b) thermocline, (c) bottom of thermocline – 300m, (d) 300 – 500m and (e) 500 – 1000m.
Chaetognatha composition
During the study period, a total of 25 species belonging to four genera were observed in the study area of which 12 species were distributed throughout the water column in varying density (Table 1). The number of species varied along different depth layers and it was relatively higher in the upper two layers (23 and 21 in MLD and thermocline, respectively) than the layers below (17 each in BT-300m, 300-500m and 500-1000m). Similar to other seasons, Sagitta was the dominant genus in most of the layers and their percentage contribution varied between 36.1% (500-1000m) to 92.4% (BT-300m). The number of species belonging to genus Sagitta ranged between 11 (500-1000m) to 19 (MLD). Pterosagittadraco was present throughout the water column though their abundance gradually decreased towards deeper waters. Krohnittapacifica and K. subtilis were observed throughout the water column whereas the three deeper water species belonging to genus Eukrohnia were mostly observed below 300 m. The dominant species were Sagitta enflata (mixed layer depth and thermocline), S. decipiens (BT-300 m and 300-500 m) and Eukrohniafowleri (> 500 m).
|
Species |
MLD |
Thermocline |
BT-300m |
300-500m |
500-1000m |
|
Pterosagittadraco |
666 |
153 |
6 |
5 |
<1 |
|
Krohnittapacifica |
1314 |
202 |
26 |
8 |
2 |
|
K. subtilis |
1473 |
250 |
29 |
5 |
4 |
|
Eukrohniabathypelagica |
0 |
0 |
0 |
0 |
9 |
|
E. fowleri |
24 |
0 |
<1 |
9 |
67 |
|
E. hamata |
0 |
0 |
0 |
<1 |
13 |
|
Sagitta bedfordii |
258 |
19 |
8 |
0 |
0 |
|
S. bedoti |
10 |
18 |
0 |
0 |
0 |
|
S. bipunctata |
1039 |
130 |
2 |
1 |
0 |
|
S. decipiens |
1471 |
831 |
444 |
94 |
27 |
|
S. enflata |
5209 |
1089 |
48 |
13 |
6 |
|
S. ferox |
1986 |
331 |
32 |
2 |
5 |
|
S. hexaptera |
1437 |
245 |
8 |
4 |
1 |
|
S macrocephala |
112 |
1 |
5 |
1 |
<1 |
|
S. maxima |
24 |
0 |
0 |
2 |
4 |
|
S. minima |
321 |
70 |
1 |
0 |
0 |
|
S. neglecta |
4617 |
713 |
64 |
10 |
5 |
|
S. oceania |
98 |
7 |
0 |
0 |
0 |
|
S. pacifica |
998 |
662 |
45 |
11 |
3 |
|
S planktonis |
8 |
4 |
5 |
0 |
0 |
|
S. pulchra |
1 |
5 |
0 |
0 |
0 |
|
S. regularis |
2761 |
412 |
81 |
8 |
3 |
|
S. robusta |
1502 |
212 |
16 |
<1 |
1 |
|
S. tasmanica |
12 |
13 |
0 |
0 |
0 |
|
S. zetesios |
9 |
1 |
0 |
1 |
<1 |
|
Unidentified |
246 |
125 |
1 |
3 |
3 |
Table 1 The abundance of chaetognath species [ind (1000 m)-3] at different depth strata
Abundance weighted values
Figure 8 depicts the species specific AO and AT values, explaining the optimum preference and optimum tolerance for different physico-chemical variables. Though temperature ranged between (6.7-26.7°C), the AO for temperature was >20°C for most of the chaetognaths (20 species). The bathypelagic species of the genus Eukrohnia preferred relatively higher salinity (AO for salinity≥34.5). Although a thick layer of oxygen minimum zone (>50% of the 1000 m water column) existed in the study region, most of the chaetognaths (15 species) showed higher optimum values for dissolved oxygen (> 3.75 mll-1).
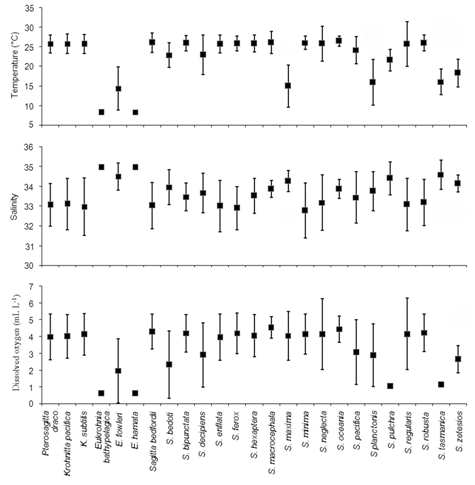
Figure 8 The species specific abundance-weighted optimum and tolerance values for the physico-chemical variables.
The ATI values in Table 2 show the variation in species-specific response for different physico-chemical variables. The ATI values for temperature varied from 0.08 (E.bathypelagica) to 1.94 (S. Planctonis). In the case of salinity, it ranged from 0.01 (E. hamata) to 1.61 (Krohnitta subtilis). The ATI values for dissolved oxygen were negligible in S. pulchra,and S. tasmanica (<0.01) whereas the maximum value was observed in S. regularis (1.78). The ATI values for all three variables were high (>1.4) only in the case of S. neglecta, and S. regularis.
|
Species |
Temperature |
Salinity |
Dissolved Oxygen |
|
Pterosagittadraco |
0.76 |
1.18 |
1.14 |
|
Krohnittapacifica |
0.83 |
1.43 |
1.08 |
|
K. subtilis |
0.83 |
1.61 |
1.04 |
|
Eukrohniabathypelagica |
0.08 |
0.01 |
0.08 |
|
E. fowleri |
1.84 |
0.76 |
1.6 |
|
E. hamata |
0.11 |
0.01 |
0.06 |
|
Sagitta bedfordii |
0.83 |
1.28 |
0.87 |
|
S. bedoti |
1.05 |
0.98 |
1.68 |
|
S. bipunctata |
0.67 |
0.77 |
0.92 |
|
S. decipiens |
1.69 |
1.11 |
1.6 |
|
S. enflata |
0.76 |
1.42 |
1.16 |
|
S. ferox |
0.65 |
1.2 |
1.01 |
|
S. hexaptera |
0.73 |
0.97 |
1.05 |
|
S. macrocephala |
0.95 |
0.47 |
0.54 |
|
S. maxima |
1.8 |
0.59 |
1.21 |
|
S. minima |
0.57 |
1.54 |
1 |
|
S. neglecta * |
1.5 |
1.55 |
1.75 |
|
S. oceania |
0.44 |
0.52 |
0.66 |
|
S. pacifica |
1.17 |
1.43 |
1.6 |
|
S. planctonis |
1.94 |
1.09 |
1.56 |
|
S. pulchra |
0.93 |
0.91 |
<0.01 |
|
S. regularis * |
1.9 |
1.47 |
1.78 |
|
S. robusta |
0.66 |
1.29 |
0.93 |
|
S. tasmanica |
1.09 |
0.81 |
<0.01 |
|
S. zetesios |
1.18 |
0.46 |
0.68 |
Table 2 The species specific abundance-weighted tolerance indices for the physico-chemical variables
*indicates significant variation (P < 0.05) in the paired t-test.
Among the 25 species recorded during the study, S. neglecta, and S. regularis were the only two species, that exhibited significant DVM between the sampled depth layers (P<0.05), clearly indicating the restricted DVM in this region (Table 2). Though, eleven species were observed in the total water column during this season, no other species showed significant DVM. Several species, which showed higher index values of only one or two variables, did not show significant DVM.
Our approach to understand the DVM process using the abundance-weighted tolerance index value displays a simple, but a reasonably accurate method to identify the possibility of any particular organism to perform DVM in TrE. In TrE, as found in our study, the wide ranges in the physico-chemical variables in upper 1000 m demand a high tolerance for the organisms to have an active migration tendency. Though the major driving force for the DVM may differ from species to species, as found in several earlier studies,31,32 in TrE, the major challenging factor for organisms is to be tolerant to this wide range of the physico-chemical variables. This, in turn, results in restricted vertical migration of the organism present in the system. Our recent observation on insignificant DVM of the total chaetognath community in the oxygen-depleted water in the northeastern Arabian Sea, another important zone in TrE,33 further corroborate this view. Among the 25 species observed, eleven species were present throughout the water column in varying density, but only two of them performed significant DVM, which clearly opposes the general concept of DVM as a common phenomenon among zooplankton in this environment.34,35
The results of the study point out that the species possessing relatively higher ATI values for the major physico-chemical variables in the system compared to their fellow species will have a higher chance to perform DVM. As observed, DVM performing S. neglecta and S. regularis, were the only two species which showed relatively higher ATI values for all three variables. S. enflata and S.decipiens, which were dominant in the epi and meso-pelagic layers, did not exhibit significant diel variation. The low ATI values for temperature in S. enflata and for salinity in S. decipiens, might be the possible reason for this. None of the meso and bathy-pelagic species of the genus Eukrohnia showed significant DVM. Their sensitivity to most of the variables (Table 2) might have restricted their vertical migration. Similarly, epipelagic species such as S. bedoti, S. bedfordii, and S. minima had low ATI values for most of the variables and were incapable of significant DVM.
Among the techniques employed to identify the DVM process, the acoustic method using the acoustic Doppler current profiler has become successful to demonstrate the signals of DVM in marine environment.4,17 But using this acoustic signal, it is not possible to identify the species-wise trend of DVM or to predict the species capable of performing DVM. Although the traditional use of the vertical profile of density values can illustrate the difference of abundance with time, it cannot predict the species capable of DVM. Interestingly, the abundance-weighted approach considers both the number of organisms and the range of the variables where they are present and thus gives a better nsight to the tolerance limit and migration tendency of the organism and thus can be an efficient tool in identifying the species capable of performing DVM among zooplankton in any tropical basin.
The vertical migration of zooplankton has a pivotal role in the efficient functioning of the biological pump, as it helps in transport of organic carbon36,37 and nitrogen flux38 produced in the surface into the deep ocean. Hence, the restricted DVM observed in our study will affect the active downward transport of organic and inorganic carbon and nitrogen in TrE, and this will lead to weak efficiency of the biological pump in this region. Climatic changes over decadal to multi-decadal scale have been reported in different parts of the marine system. The warming of sea surface temperature39,40 will result in the increase of the temperature gradient between the surface and mid-depth water. Similarly, the expansion of oxygen minimum zone in the mid-depth water of TrE41,42 will also facilitate the formation of sharper gradients in the dissolved oxygen values between the depth layers. As found in our study, that, for successful DVM, the organisms have to be capable enough to withstand the wide range of the physico-chemical variables and the increasing trend in the ranges of these variables in response to the climate changes will thus make it more difficult for organisms to perform DVM in tropical oceans. Though, zooplankton has been mostly reported to migrate from near-surface region to 400 - 600 m depth or more in the open ocean region,38,43 in TrE, due to sharp vertical gradient in physico-chemical variable and the increasing tendency of the gradients due to climate changes will thus either reduce the tendency of DVM or restrict the vertical migration within shallow depth range.
We thank the Director, National Institute of Oceanography and the Director, Centre for Marine Living Resources and Ecology, Kochi for providing the facilities. We are grateful to the captain, crew, and scientific members of FORV Sagar Sampada for the help rendered in sampling. This study was carried out under the program Marine Research on Living Resources funded by the Department of Ocean Development, Govt. of India. The 1st author is thankful to CSIR for the award of post-doctoral research associate. This is NIO contribution
None.

©2017 Karati, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.