Journal of
eISSN: 2378-3184


Research Article Volume 4 Issue 6
1Stazione Zoologica Anton Dohrn IME Department, Benthic Ecology Center, Italy
2Dipartimento di Biologia, Universit
3CNRInstitute of Biosciences and Bioresources, National Research Council, Italy
Correspondence: V Zupo, Stazione Zoologica Anton Dohrn IME Department, Benthic Ecology Center, Italy
Received: November 18, 2016 | Published: December 15, 2016
Citation: DOI: 10.15406/jamb.2016.04.00100
The trophic adaptability of a species may influence its dispersion potential and the ability to invade foreign territories. Understanding the factors that facilitate trophic adaptability may help the provision of forecasts about the potential dispersion of allocthonous species, even in a warmer and acidified world, according to the current trends of global changes. Various studies demonstrated the adaptability of Penaeus japonicus Bate to variable feeding regimes under natural conditions. To optimize artificial diets for the aquaculture of Penaeid shrimps, gut content data of specimens cultured in ponds were compared to contents from shrimps fed on natural macro benthic communities in a brackish-water lagoon. In addition, the feeding adaptability of this shrimp to scarcely diversified benthic associations was tested in aquaculture ponds. Our comparative analyses confirm that P. japonicus feeding pattern may be largely adapted to variations in the available benthic organisms, in different management conditions.
Keywords: Penaeus japonicus, Food, Brackish water, Culture, Adaptability, Marsupenaeus japonicus
Penaeus japonicusBate 1888, the “Kuruma shrimp”, whose taxonomical identity is still debated Tsoi et al.1 is an ideal species for aquaculture purposes, due to its adaptability to different environmental conditions Lumare.2 The species is still indicated by means od two scientific names, i.e., P. japonicus and Marsupenaeus japonicus, due to a controversy in the status of the genus Penaeus Fabricius, 1798 (see, for example, Pérez Farfante & Kensley Ma et al.3,4 Both names are still used by various organizations and databases, eg. WoRMS (World Register Marine Species), FAO, DAISIE (Delivering Alien Invasive Species Inventories for Europe);however, in this study we will follow the most updated results Tsoi et al.1 for the definition of Penaeus and hence we will use the current name Penaeus japonicus Bate 1888.
Penaeus japonicus exhibits attractive brownish-red bands and it is named “the kuruma shrimp” because of a characteristic wheel-like banding pattern. It is economically important for the world shrimp market due to an easy management of pond cultures and the fast grow-out.5 Many aspects of its physiology and reproductive biology have been clarified.6,7 but the available information on its trophic needs in intensive and semi-extensive culture conditions is still incomplete. Previous studies identified the feeding patterns of P. japonicus under natural conditions, after its introduction in coastal lagoons.8-11 In fact, this species appears to be able to easily invade new territories when accidentally introduced in natural environments.12-13 and the global increase of temperatures in natural basins, even in the Mediterranean area, facilitates its dispersion.14
At present, Penaeus japonicus is widely distributed in Japan, the South China Sea, Korea, the Archipelago of Malay, the Red Sea, the northern coast of Queensland in Australia, and the western Indian Ocean, up to the eastern South Africa.3 However, according to Galil & Zenetos.13 it was considered as a Lessepsian migrant in the Mediterranean waters since 1924. Obviously, its successful adaptation in the Mediterranean may threaten indigenous species, as P. keraturus and other crustacean decapods, as well as its main prey, represented by molluscs and polychaetes.15
A dynamic feeding model was demonstrated for this species in relationship to the predation on natural benthic populations.16 This evidence allows us for predicting a destructive influence of P. japonicus on wild ecosystems as well as a certain degree of adaptability to benthic communities characterized by low species diversity, as those characterizing the culture ponds. Therefore a study comparing natural feeding patterns with those observed in prawns managed in extensive and semi-intensive conditions was performed by means of gut contents analyses. The results of the present study, in fact, may allow from one side an optimization of inexpensive diets for this species in aquaculture ponds and, on the other side, to explain some of the factors promoting the adaptability of a given species when introduced in new areas.
Five ponds were set in Cammarata (Lesina, Southern Italy) (Figure 1). The first 4 ponds (100 m2 each one) were managed in semi-extensive conditions, at density of 2.5; 1.5; 2.5; 1.5 post larvae (PL22) per square meter, respectively; the fifth one (200 m2) was managed in extensive conditions, at a density of 12 post-larvae (PL22) per square meter.
Water was partially changed daily, by pumping from the adjacent lagoon, in order to promote correct environmental conditions for the grow-out of the shrimps.17 and the development of benthic fauna and flora driven by larvae and various organisms present in the lagoon. The finest rate of changes was determined by measuring selected chemical and physical parameters, i.e., temperature, salinity, pH, dissolved oxygen, dissolved nutrients. In particular, changes were performed to assure consistence of the above-mentioned parameters in comparison to the natural ones, measured in the lagoon.18 To perform the gut content analyses, three collections of shrimp were obtained in the early night from each pond, to obtain individuals containing undigested prey. The first sample refers to shrimps aged 6 weeks, the second one refers to shrimps aged 12 weeks, and the last one refers to adults sampled a few days before the end of the experiment.
Samples were immediately transferred in the laboratory and deep-frozen. Each specimen was measured (total length), weighed, and finally sacrificed, fixed and dissected for the analysis of the gut contents. A presence-absence matrix was compiled, taking into account each specimen sampled and each food item identified in its stomach, according to Zupo et al.2 Data were managed by means of a MS-Excel spreadsheet and compared with those obtained by previous authors.9,11,16 using Graph pad Prism software. In particular, the following indices were taken into account:
Numeric frequency, obtained on the basis of the following relationship
Np= number of individuals of the food item "i"
Tp= Total number of individuals, of all food items, found in the gut.
Prey frequency, obtained on the basis of the following relationship
Nsp= number of guts containing the item "i"
Ns= number of "non-empty" stomachs studied.
Vacuity coefficient, obtained on the basis of the following relationship
Nv= number of empty guts recorded.
Nt= total number of guts studied.
Previous authors (Piscitelli & amp;Scalera Liaci, 1983) classified each prey, on the basis of their frequency index (Fp), in the following categories:
In order to obtain quantitative information about the trophic needs of shrimp of different ages, the feeding index "FI" .19 was also calculated; it is computed on the basis of the following relationship:
PCS = weight of the gut contents.
PTA = weight of the animal body.
The average total length of individuals sampled in all ponds, in the three samples were, respectively 9.4, 13.4 and 15.0 cm (Table 1). The analysis of the gut contents, classified according to larger food categories (Table 2) permits a direct comparison with the data obtained in brackish water wild environments.11 The preferential food items in all samples were Annelid Polychaetes (Fn=16.7%), insect larvae (Fn=12.7; represented mainly by Chironomid Dypters), Molluscs (Fn=15.3), and Crustaceans (Fn=19.9; represented mainly by Amphipods and Copepods). Large amounts of artificial food (Fn=12.4) were found in prawns cultured in semi intensive conditions. Other food items, less important, were macroalgae (Fn=14.0), Foraminiferids (Fn=1.3) and Isopod Crustaceans (Fn=1.3).
|
Sample 1 |
Sample 2 |
Sample 3 |
|
|
Mean length |
9.39 |
13.42 |
15.05 |
|
Mean weight |
5.72 |
15.72 |
25.19 |
|
Gut weight |
0.05 |
0.13 |
0.16 |
|
Cv |
13.3 |
12.5 |
25.9 |
|
FI |
0.55 |
0.18 |
0.08 |
Table 1 Main biometric and trophic parameters of the three samples investigated
|
Prey categories |
Fn |
Fp |
||
|
This study |
Lagoon |
This study |
Lagoon |
|
|
Foraminiferids |
1.3 |
1.1 |
5.6 |
5.5 |
|
Anellides |
16.7 |
7.2 |
61.1 |
22.9 |
|
Perinereis cultrifera |
2.3 |
|||
|
Polydora ciliata |
13.9 |
59.2 |
||
|
Nereidae |
4.2 |
|||
|
Polynoidae |
0.7 |
|||
|
Others |
2.2 |
9.2 |
||
|
Molluscs |
15.3 |
26.5 |
55.6 |
43.1 |
|
Hydrobiasp |
1.3 |
10.3 |
3.7 |
|
|
Cerastoderma edule |
3.4 |
|||
|
Cerastoderma glaucum |
2.6 |
11.1 |
||
|
Haminea navicula |
0.9 |
|||
|
Abra ovata |
11.3 |
48.1 |
||
|
Crustaceans |
19.9 |
32.4 |
74.1 |
48.2 |
|
Ostracodes |
0.1 |
|||
|
Misidaceans |
0.1 |
|||
|
Copepods |
7.4 |
31.5 |
||
|
Amphipodes |
11.3 |
48.1 |
||
|
Corophium acherusicum |
0.4 |
|||
|
Corophium insidiosum |
1.3 |
10.7 |
7.4 |
|
|
Gammarides |
10.6 |
0.7 |
||
|
Gammarusa equicauda |
10.6 |
2.3 |
||
|
Microdeutopus gryllotalpa |
1.5 |
|||
|
Isopods |
1.3 |
3.7 |
||
|
Spheromides |
1.3 |
3.7 |
||
|
Cyathuracarinata |
6.4 |
|||
|
Idotea baltica |
1.4 |
|||
|
Cymothoidaae |
0.1 |
|||
|
Decapods |
||||
|
Palemonetesantennarius |
0.9 |
|||
|
Palaemonelegans |
0.2 |
|||
|
Palaemonidi |
0.3 |
|||
|
Insects |
21.7 |
12.5 |
88.9 |
18.3 |
|
Chironomides |
20.8 |
12.5 |
88.9 |
|
|
Others |
1.3 |
5.6 |
||
|
Eggs |
1.3 |
5.6 |
||
|
Fish |
18.3 |
36.2 |
||
|
Sygnathusabaster |
7.2 |
|||
|
Macrophytes |
14 |
42.6 |
6.4 |
|
|
Blidingiasp |
1.7 |
5.6 |
||
|
Cladophorasp |
4.7 |
20.4 |
||
|
Chaetomorpha gracilis |
4.7 |
20.4 |
||
|
Chaetomorphacapillaris |
2.6 |
11.1 |
||
|
Artificial foods |
12.4 |
74.2 |
Table 2 Prey frequency (Fp) and numeric frequency (Fn) data of gut contents of Penaeus japonicus cultured in Cammarata, compared to those of populations living in the Lesina lagoon
The comparison of the numeric frequencies of the main taxa found in the gut contents of individuals sampled in culture ponds (present study) and in natural environments Piscitelli & Scalera Liaci.9 demonstrated that the trophic importance of some taxa changed dramatically under different environmental conditions, according to the age of prawns. In particular, insects and polychaetes were clearly prevalent food items in culture conditions, while a higher variability in prey taxa was observed in natural conditions. Molluscs (mainly bivalves) were a preferential prey in both natural and culture conditions. A few taxa were found in the guts of individuals collected in pond algae were represented by four species, i.e., Blidingia sp, Cladophora sp, Chaetomorpha gracilis and C. capillaries, polychaetes were represented only by Polydoraciliata, bivalve Molluscs were represented by Cerastoderma glaucum, Abraovata and, at lower abundance, by Mytilastermarioni a few gastropods (Hydrobia sp) were found in the gut contents of cultured individuals; amphipods were represented by Gammarusa equicauda and, more rarely, by Corophium insidiosum isopods were represented by a few individuals of Sphaeromids.
The shrimp feeding activity showed a decrease in the last sample. FI values of 0.55, 0.18 and 0.08 were recorded through the three samples, respectively (Table 1), according to the mean temperatures of 28.5, 26.7 and 17.9, measured during the experimental period. Chironomid dypters accounted for the highest Fp values (88.9%) calculated on the total sampled individuals (Table 2), followed by Polychaetes (61.1%) and Molluscs (55.6), in agreement with the results obtained in the wild by Piscitelli & amp;Scalera Liaci.9 The vacuity coefficient was very low (13.3%, 12.5% and 25.9% in the 3 samples) in comparison with the results of previous authors and it is close to the frequency of individuals in ecdisys. A decrease of FI and an increase of Cv indices were detected during the grow-out period in all ponds (Figure 2). Striking differences were observed among the prey frequencies measured in this study (in culture ponds) and in the natural environments. For example, algae, Annelid polychaetes and insects exhibited significantly higher frequencies in the guts of cultured animals in comparison to the wild, while mollusks and crustaceans showed more complex patterns and higher numeric frequencies in the wild (Table 2). However, the abundance of various taxa varied with the age, as younger individuals preferred Crustaceans and small mollusks, while older individuals fed mainly on macrophytes and annelid polychaetes (Table 3).
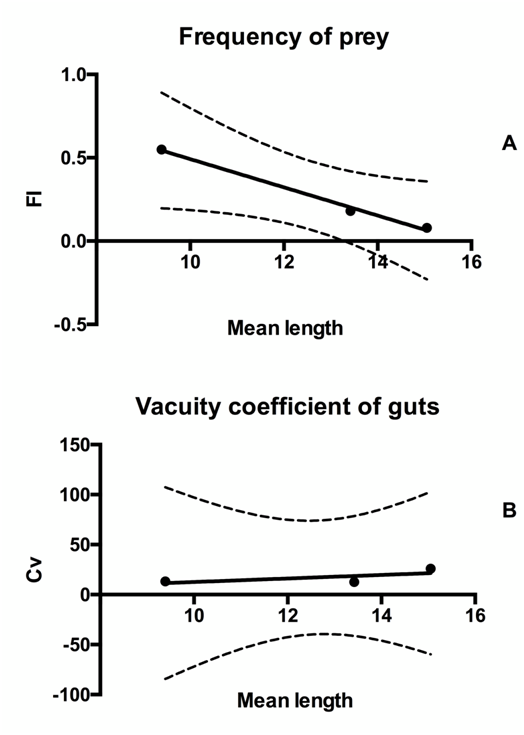
Figure 2 Comparison between the feeding indices considered in the present study; a progressive decrease of the FI index (A) and a slight increase of Cv index (B) is shown, according to the size of the prawn.
|
Lesina Lagoon |
This study |
|||||
|
Size |
10 cm |
12-14 cm |
14-16 cm |
10 cm |
12-14 cm |
14-16 cm |
|
Foraminiferids |
22.2 |
1.9 |
3.4 |
1 |
1.2 |
|
|
Anellides |
16.7 |
25 |
23.7 |
11.3 |
16 |
20.7 |
|
Crustaceans |
50 |
50 |
44.1 |
29 |
14 |
20.7 |
|
Molluscs |
44.4 |
46.2 |
37.3 |
25 |
15 |
10.3 |
|
Chironomids |
16.7 |
13.5 |
27.1 |
27.2 |
20 |
22.1 |
|
Fish |
23.3 |
50 |
33.9 |
- |
- |
- |
|
Macrophytes |
- |
5.8 |
11.9 |
6.8 |
16 |
15.6 |
|
Artificialfoods |
- |
- |
- |
- |
17 |
7.8 |
Table 3 Numeric frequency (%) of taxa recorded in the gut contents of Penaeus japonicus at different size classes. Data from the present study are compared to those obtained by previous authors Piscitelli & Scalera Liaci.9 in the Lesina Lagoon.
In general, Crustaceans and mollusks were more abundant in field-collected shrimps, while insects and annelids were more abundant in cultured individuals, that also exhibited an almost equal abundance of the main food items (Figure 3). The prey frequency index also exhibited variations according to the size when the shrimps collected in the lagoon of Lesina were compared to those collected in pond (Figure 4). In particular, Foraminiferids were almost absent in the cultured shrimps while they were abundant in the youngest individuals collected in the wild. Chironomids were fed especially by larger individuals in the field, while they were used by all size classes in the pond cultures. Macrophytes were mainly consumed by older individuals in the field and their consumption increased, in the tanks, also in younger shrimps (Figure 4a&4b).
The qualitative analyses of the gut contents confirmed the "opportunistic-carnivore" feeding habit of Penaeus japonicas and, in general, the adaptability of various dietetic regimes characterizing penaeid shrimps.20 however, its feeding preferences varied mainly according to the age.21 The decrease of the feeding activity (FI index) observed in this study was probably due more to the decrease in temperature.22 than to the decrease of the abundance of benthic prey. In fact, the presence of an artificial food should replace any lack of prey.23 Fn and Fp indices calculated in the present study, compared to those obtained for individuals sampled in the wild, showed different feeding patterns (Table 2). A comparison of the data collected in the present work for three size classes (corresponding to the three subsequent samples), with those of similar size classes sampled in the natural environment.9,24 demonstrates prey preference exhibited by prawns in natural conditions, as Crustaceans, Polychaetes and fishes were the most consumed categories. They were substituted by insects, Molluscs and artificial foods (pellets) under culture conditions, as demonstrated in this investigation.
However, it was demonstrated that Polychaetes are important also for the reproduction of this species.25 and therefore the presence of these prey items could be fundamental in culture ponds. Foraminiferids were an important food item for young individuals sampled in the natural environment.9 However, they were very quite rare in the guts of cultured individuals. This difference may be due to the low abundance of this prey in the benthic population of the culture ponds. A similar conclusion is reached when prey fish data are analyzed fish were consumed by wild individuals, but they were absent in the culture ponds. These relationships confirm that P. japonicus adapts easily to any environment and it is able to exploit low-diversity benthic food associations, as those observed in culture ponds.
Arthropods and Molluscs could play an important role in the trophic ecology of this decapod, especially at larger sizes of the prawn; young individuals (< 10 cm total length) cultured in semi-intensive conditions showed a higher preference for Chironomid insects and algae, than individuals sampled in the wild. The frequency of Chironomid insects in the guts of specimens in culture conditions, higher than in natural conditions, could result from an higher abundance of these items in culture ponds; however, these items should be considered as “preferential prey” (Fp>50%). It is worth to observe that also "non-living" organic matter can be exploited by the prawn, given the high values of feeding indices yielded by pellet foods (Fn= 12.4; Fp= 74.2).
Therefore, P. japonicus is adaptable to different feeding regimes.26 and it can exploit prey found in a range of environmental conditions. Interestingly, several aspects of its feeding biology were based on single or a few observations.27 As demonstrated by the present study, its dietetic pattern is complex and not constant during the development, but variable also according to the culture conditions. This conclusion confirms the importance of P. japonicus for aquaculture purposes and its potentially destructive power for the wild environments. In fact, the ability to exploit many food items may facilitate its life in culture ponds, but it may represent as well a danger for the stability of native macro benthic associations when P. japonicus is accidentally introduced into the wild.28
Linguistic and scientific comments by G. Kraemer improved the original manuscript. The authors are indebted to the Benthic Ecology group (Stazione Zoologica Anton Dohrn) for the help in taxonomical analyses and to G. Casolino, V. Marolla and V. Biscotti for the invaluable help given in sampling and analysis of biological and chemical data.
None.

©2016 , et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.