Journal of
eISSN: 2378-3184


Research Article Volume 4 Issue 4
Department of Aquaculture, College of Fisheries (KVAFSU), India
Correspondence: Shashikant J Meshram, Department of Aquaculture , College of Fisheries (KVAFSU), Kankanady, Mangalore, India
Received: July 06, 2016 | Published: September 21, 2016
Citation: Meshram SJ, Murthy HS, Swain HS, Pai R, Ali A, et al. (2016) Preliminary Study of the Effect of Dietary Beta-Glucan on the Gut Morphology of Giant Freshwater Prawn, Macrobrachium Rosenbergii. J Aquac Mar Biol 4(4): 00090. DOI: 10.15406/jamb.2016.04.00090
The experiment was carried out on giant freshwater prawn, Macrobrachium rosenbergii in triplicate for 60 days with a dietary supplementation of beta-glucan to evaluate the effect on gut morphology. Juveniles of prawn (with initial weight of 0.34 ± 0.01 g) were fed with beta-glucan incorporated diets at levels 0, 1, 2 and 3 g/kg of feed. After 60 days the gut micrographs revealed that there were higher goblet cells, microvilli length, crypt and absorption area observed in the treatment of 1 g/kg beta-glucan incorporated diet, however it was not significant as compared to other treatments. Less developed in control and disturbed structure were observed in levels of 2 and 3 g/kg of beta-glucan incorporated diets. The results revealed that the diet incorporated with 1 g/kg of beta-glucan showed development of microvillus structure and goblet cells. However higher doses of beta-glucan showed negative effects on gut morphology of Macrobrachium rosenbergii juveniles fed for 60 days.
Keywords: Beta-glucan, Gut morphology, Macrobrachium rosenbergii
The scampi is native to Southeast Asian countries and being cultured in India, China, Bangladesh, Vietnam, Malaysia, Thailand, Taiwan, Brazil, Ecuador, and USA. But now a day’s its culture decline day by day due to major constraints like diseases, feed cost, seed availability, water quality and inadequate nutrition are responsible for fluctuation of M. rosenbergii production. The dietary supplementation of proper nutrients helps in improving growth, survival and gut immunity of prawn. In recent day’s attention has been paid for use of some nutraceuticals products derived from yeast like β-glucan, nucleotide, mannan oligosaccharide (MOS) and organic selenium as dietary supplements, which helps in promoting growth and survival in fish and shellfish.1,2 Also use of these types of prebiotics will helpful to minimize the use of antibiotics in aquaculture, which will be serving as one of the solution for eco-friendly aquaculture.
Beta-glucan is a polysaccharide derived from the cell wall of yeast and it has been used as prebiotics in poultry and swine husbandry and in aquaculture.3-5 It is demonstrated that the lactic acid bacteria (e.g. Bifidobacterium, Lactobacillus) have the ability to tolerate the acidic and bile environment of the intestinal tract. Lactic acid bacteria (LAB) also functions to convert lactose into lactic acid, thereby reducing the pH in the GIT and naturally preventing the colonization by many bacteria.6,7 Gastric bacterial populations may also play an important role with regard to immunostimulation and development of gut-associated lymphoid tissues.8
The importance of gut morphology have been explained in relation to scavenger organ9,10 balanced and dynamic interactions among mucus layers, intestinal epithelial cells, micro-biota and host immune defense.11 Also the disruption in the intestinal homeostasis results in the defective mucus barrier with increased permeability that results in inflammation and injury of the intestinal mucosal cells.11,12 There is little literature on effect of beta-glucan on gut morphology of fish and shellfish. Realizing the importance of nutraceutical in prawn diets, the research theme was conceptualized with objective to evaluate the effect of β-glucan on gut morphology of giant freshwater prawn Macrobrachium rosenbergii.
The experiment was carried-out in triplicate for 60 days with a dietary supplementation of beta-glucan. Juveniles of M. rosenbergii (with initial weight of 0.34 ± 0.01 g) were fed with beta-glucan incorporated diets at levels 0, 1, 2 and 3 g/kg of feed. After 60 days of the experiment, three juveniles of prawn from each replicated treatment groups were selected and dissected out to get the gut tissues. The fixed tissues were processed using an automatic tissue processor (Shandon, Citadel 1000, England) and embedded in paraffin wax (Shandon, Histocenter 2, England). Sections were cut at 5-6 µm thickness (Weswox Dptk MT-1090A, India) and stained with haematoxylin and eosin for general morphological purposes. The tissue slides were prepared as per protocol.12 Slides were documented photographically with Olympus CX 41, Japan attached to microscope and PC. The images of different treated groups were visually analyzed and compared for the results.
Light microscopy of gut
The gut morphology of prawns fed on β-glucan supplemented diets is shown in Figure 1. After 60 days of experiment there were higher goblet cells, microvilli length, crypt and absorptive area observed in 1 g/kg beta-glucan incorporated diet group as compared to 2 g/kg, 3 g/kg and control groups. Whereas less developed structure in control group. However disturbed structure, higher infiltrations in cells and ruptured villi structure were observed in 2 g/kg and 3 g/kg beta-glucan incorporated diet groups.
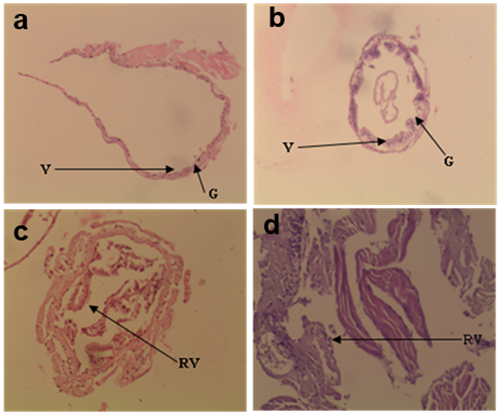
Figure 1 Light micrograph on gut morphology (T. S.) of Macrobrachium rosenbergii juvenile, fed diets containing 0.0, 1.0, 2.0, and 3.0 g/kg β-glucan, which are depicted as A-control, B-1.0, C-2.0 and D-3.0 treatments. (V- villi, G- goblet cells, RV- ruptured villi) [haematoxylin and eosin (H & E) x10].
The intestine is made up of a single type of epithelial cell and is considered as a scavenger organ9 and plays role in host immune defense system through gut.11,12 There is little literature on effect of beta-glucan on gut morphology of fish and shellfish. Kuhlwein et al.14 observed the ultrastructural changes and microbial communities in intestine by dietary supplementation of β (1, 3) (1, 6)-D-glucan (MacroGard) showed modulation in microbial communities and influences the morphology of the apical brush border of mirror carp, Cyprinus carpio. The channel cat fish, Ictalurus punctatus fed on 0.1 and 2% dietary supplementation of yeast polysaccharide (β-glucan) showed significantly increased intestinal fold height and goblet cells.15 Kuhlwein et al.14 observed that there was no significant difference in intestinal absorptive area and number of goblet cells in either intestinal region of mirror carp, Cyprinus carpio fed with dietary supplemented β (1,3) (1,6)-D-glucan (MacroGard). Also observed significantly higher infiltration of leucocytes into the epithelial layer of Cyprinus carpio fed diets supplemented with 1% and 2% MacroGard (β (1,3) (1,6)-D-glucan) in the anterior intestine compared to fish fed the control and 0.1% MacroGard. Olsen et al17 reported the damaging effect of dietary prebiotics (inulin) on intestinal enterocytes in Arctic charr, Salvelinus alpines.
In the present study the gut morphology of juvenile prawn fed with 1 g/kg beta-glucan supplemented diet group showed higher goblet cells, crypt, unification and microvilli length among the treated groups, but it was not significant as compared to other groups (Figure 1). Also showed higher absorptive area, which ultimately resulted in higher growth (weight gain) than other groups. Whereas, 2 and 3 g/kg beta-glucan incorporated diet groups showed higher infilteration in cells and ruptured villi structure, may be due to excess dose of beta-glucan which accumulates in enterocytes cells of epithelial layer of intestinal region16,17 and control group showed less developed (goblet cells, crypt and microvilli) respectively, it may be due to less developments of microorganisms in gut and goblet cells.18,19 The histological gut morphology observed under present study are in agreement with the literature cited above, which suggested that prawns fed with 1 g/kg beta-glucan incorporated diet found suitable with higher crypt, unification and microvilli length and without any detrimental effect on gut morphology of M. rosenbergii juvenile.
From the results it can be concluded that, 1g/kg beta-glucan incorporated diet plays role in improving goblet cells, microvilli length and absorption area in the gut of giant freshwater prawn, Macrobrachium rosenbergii for 60 days, without any detrimental effect.
None.
None.

©2016 Meshram, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.