Journal of
eISSN: 2378-3184


Research Article Volume 4 Issue 2
Department of Marine and Coastal Studies, Madurai Kamaraj University, India
Correspondence: Anand Muthusamy, Department of Marine and Coastal Studies, Madurai Kamaraj University, Madurai- 625 021, Tamilnadu, India, Tel +919-444-120-690;
Received: October 23, 2015 | Published: March 31, 2016
Citation: Priya RJ, Muthusamy A, Maruthupandy M, Beevi AH (2016) Biomarker Response of Ocean Acidification Induced Hypercapnia on Marine Bivalve Donax cuneatus, Linnaeus 1758. J Aquac Mar Biol 4(2): 00077. DOI: 10.15406/jamb.2016.04.00077
The present study reports the investigation of the sub lethal impacts of ocean acidification induced hypercapnia (pH 7.5 and 7.0) and leakage of sub sea bed Carbon Dioxide storage sites (pH 6.5-5.5) on marine bivalve Donax cuneatus, Linnaeus 1758 collected from the Gulf of Mannar province. Fifteen days experiment was carried out in laboratory microcosms in a closely monitored environment. The impacts of Hypercapnia on Biomarker enzymes indicating neurotoxicity (Acetylcholinesterase), oxidative stress (lipid peroxidation and catalase) and phase II biotransformation of xenobiotics (glutathione S transferase, and reduced glutathione) were estimated. The increase in lipid peroxidation and catalase activities suggest hypercapnia induced oxidative stress. The decreasing pH also increased the activity of reduced glutathione and glutathione S transferase which acts as detoxifying enzymes for Hypercapnia induced toxicity however the hypercapnic conditions inhibited the levels of acetylcholine esterase which indicates the polluted environment substantiating stress induced high rate of mortality at low pH levels. The above used biomarkers thus provide a valuable tool for monitoring ocean acidification induced hypercapnic stress at a sublethal level.
Keywords:Ocean acidification, Hypercapnia, Microcosm, Donax cuneatus, Biomarkers
AchE, Acetylcholine Esterase; ACTI, Acetylcholine Iodide; ANOVA, Analysis of Variance; BSA, Bovine Serum Albumin; CAT, Catalase; CDNB, Chlorodinitrobenzene; CO2, Carbon Dioxide; DO, Dissolved Oxygen; DOE, Department of Energy; DTNB, 5,5 Dithiobis - (2 Nitrobenzoic Acid); fCO2, Fugacity of Carbon Dioxide; GSH, Glutathione; GST, Glutathione - S - Transferase; H2O2, Hydrogen Peroxide; ICES, International Council for the Exploration of the Sea; IPCC, Intergovernmental Panel on Climate Change; MDA, Malonedialdehyde; OA, Ocean Acidification; pCO2, Partial Pressure of Carbon Dioxide; pH(T), Total Hydrogen Scale pH; R2, Regression Value; SD, Standard Deviation; SDS, Sodium Dodecyl Sulphate; SRES, Special Report on Emission Scenario; TA, Total Alkalinity; TBA, Thiobarbituric Acid; TCA, Trichloroacetic Acid; UV Vis, Ultra Violet - Visible; ε, Molar Extinction Co Efficient
Among the various stressors that coerce the marine environment ocean acidification plays a crucial role. Assessing the effects of such acidity on the chemistry and biology of the marine environment is substantial to interpret the
global changes over decades. pH, the basic unit of acidity in seawater has already dropped by 0.1 units which accounts to 26% increase in acidity .1,2 a further 0.3 to 0.4 units fall in pH is anticipated by 2100.3 Many measures have so far been established to moderate the emission of CO2 thereby restraining the negative impacts of OA. Geologic CO2 sequestration is one such amelioration process well established in many parts of the world.4 however these mitigation measures may lead to sub surface leakage overtime.5 there by exposing the animals to unequivocal hypercapnia much satirical than the anthropogenic acidification of the oceans.6,7
Any changes in the ocean chemistry due to sub storage leakage or other anthropogenic sources can be easily prognosticated with high certainty. What is less certain at the present juncture is the consequence of such changes on the marine animals. We know very little about the animals that scarcely encounter such spatially restricted environment. Animals that are not regularly exposed to hypercapnic condition are highly sensitive.8 There are enough research evidences that suggest that the taxa dependent on calcium carbonate for building shells, skeletons and test are the most vulnerable to these changes. Most of the research on OA so far has been centered on the survival and the biology of the marine calcifying animals.9,10
The present study here in attempts to assess the sensitive biomarker response to the sub lethal impacts of pH at 5 different levels on marine bivalve Donax cuneatus.
Animal collection and maintenance
Donax cuneatus identified after Linnaeus, 1758 were collected from the intertidal region of the Gulf of Mannar at 09°15›06.43” N latitude and 079°06’33.71” E longitude. The collected animals were immediately transferred to the Pudhumadam field research laboratory with utmost care. The animals were washed with filtered sea water to remove the adhering epiphytes and acclimated to laboratory conditions with ambient requirements. The animals were fed twice a day with mixed phytoplankton culture.
CO2 microcosm design
Microcosm facility for the exposure was designed as per the descriptions of Widdicombe and Needham .11 and Dupont et al.12 The system consisted of two chambers viz. mixing chamber and an incubation chamber. The mixing chamber with 2 liters holding capacity was used to perturb the seawater where as the incubation chamber measuring 2 liters holding capacity was used to expose the animals to the acidified sea water. Six microcosms with six different pH regimes were used in the present study. The pH regimes selected for the study are 8.1 (control or unperturbed seawater), 7.5, 7.0, 6.5, 6.0 and 5.5. The lower limits 7.5 and 7.0 were selected based upon the seacarb output and approximated the modeled decrease in surface ocean pH by 2100 under the IPCC A2 SRES scenario of CO2 emissions.13 Whereas the pH ranges 6.5 - 5.5 mimicked the environment in the sub seabed CO2 leakage sites.11
Seawater perturbation
In order to mimic the natural process of ocean acidification, the seawater was manipulated by bubbling a mixture of pure CO2 and air until the desired pH was achieved. A pH stat system that ensured 100% gas flow and cut off ability when the pH went above or below the set value was used for the study. The seawater used for the study was pumped from Pudhumadam coast and stored in the over head tank which was then manipulated in the mixing chamber by gas bubbling. Manipulated seawater was then transferred to the incubation chamber.
Medium term microcosm exposure
Ten healthy animals were randomly allocated in each incubation chamber. The animals were maintained under 12:12 hours natural irradiation process. The animals were fed with mixed plankton culture throughout the experimental period. The pH in the chamber was maintained constant by a pH stat system and the dissolved oxygen levels in the chamber was monitored and maintained at ambient level. Water was siphoned twice a day to remove the organic load. Any changes in the behavior of the exposed animals were closely monitored.
CO2 microcosm environment analysis
After allocating the animals any change in the seawater variables at constant pH was monitored at an equal interval throughout the experimental period. The pH of the water was monitored using Oakton pH 700 bench top meter, where as Oakton waterproof DO 300 meter was used for measuring DO and Comark PDQ 400 high accuracy thermometer for temperature. 100 ml of subsamples were collected in every 24 hours and subjected to TA analysis with the procedure adopted from River Watch Network .14
Mortality rate
Any sign of mortality was closely monitored throughout the experimental period. Mortality was confirmed by permanent opening of the shells. The deceased animals were immediately removed from the experimental chambers.
Biomarker analysis
Total tissue protein: Total protein concentrations were measured by Lowery et al.15 using BSA as reference standard material. Protein concentrations were expressed as mg/g wet weight of tissue.
Acetylcholine esterase activity
The activity of AchE in the tissue of the exposed animals were analysed according to the method of Ellman et al.16 The reaction mixture contained Sodium phosphate buffer, DTNB, 10mM phosphate buffer and substrate ACTI. The increase in absorbance was read at 405 and 412 nm at 5 mins interval using Systronics double beam UV - Vis Spectrophotometer 2201 series. Molar extinction co- efficient (1.36 ×104 M-1 cm-1) was used to measure the AchE activity and the results were expressed in µmols min-1 mg-1 of total protein.
Catalase activity
CAT activity was measured as per the methods of Greenwald.17 by the decrease in absorbance at 240nm due to H2O2 consumption. The difference in the absorbance per unit of time was measured as CAT activity (ε = 39.45mM-1 cm-1). Results were expressed in mmol min-1 mg-1 of total protein concentration.
Glutathione - s - transferase activity
The GST assay was performed using a modified method based on Habig et al.18 20mM CDNB was used as the substrate and 20mM GSH as the co-substrate. GST activity was estimated by measuring the formation rate of conjugated substrate (CDNB - GSH). Results were expressed in µmol min-1 mg-1 of total protein.
Lipid peroxidation
Lipid Peroxidation was determined as described by Okhawa et al.19 Briefly, the reaction mixture consisted of 0.2ml of 8.1% SDS, 1.5ml of 20% acetic acid (pH 3.5) and 1.5ml of 0.8% aqueous solution of TBA and 0.2ml of brain homogenate. The mixture was made up to 4 ml with distilled water and heated at 950° C for 60 min. After cooling with tap water, 5 ml of n-butanol and pyridine (15: 1, v/v) and 1 ml of distilled water were added and centrifuged. The organic layer was separated out and its absorbance was measured at 532nm in Systronics double beam UV- Vis Spectrophotometer 2201 series and MDA content was expressed as nmol/mg protein using molar extinction coefficient.
Reduced glutathione activity
RG was measured according to the method of Ellman.20 An equal quantity of tissue supernatant was mixed with 10% TCA and centrifuged to separate the proteins. To 0.1 ml of this supernatant, 2ml of phosphate buffer (pH 8.4), 0.5ml of DTNB and 0.4ml of double distilled water were added. The mixture was vortexed and absorbance was read at 412nm with in 15min. The concentration of reduced glutathione was expressed as μmol/g tissue using molar extinction coefficient.
Statistical analysis
The data were expressed in mean ± SD. One way ANOVA using Stat plus 2009 was performed with pH as the factor and biomarkers as the dependent variables. p<0.05 were assumed to be statistically significant. Regression analyses were also performed using Stat plus 2009.
CO2 microcosm environment analysis
Climate change and OA are evil twins totally different from each other. Climate change involves green house effect that totally alters the climate but OA on the other hand alters the chemistry of seawater. OA which is caused by the uptake of CO2 is ruled by the fCO2 along with its uptake velocity which is in turn manipulated by water temperature, wind speed and other physico chemical parameters.21 In the present study mean changes in temperature, salinity, dissolved oxygen, total alkalinity and dissolved inorganic carbon were measured to rule out the impact of increasing carbon dioxide on these parameters. Unlike the intricate reactions involved in green house effect, the physico chemical changes in ocean acidification are simple and easy to monitor in an accurate manner for a given concentration of CO2 in seawater.
In the present study the changes in the physico chemical parameters in all the microcosm chambers were monitored four times a day at equal intervals throughout the experimental period. The pH was maintained constant and significant changes (p<0.05) was observed in the temperature, salinity, alkalinity and dissolved oxygen levels. The mean variations observed in the temperature, salinity, TA and DO are summarized in Figure 1a-1d. The control chamber exhibited the lowest temperature of 27.3 ± 0.09 where as the pH 6 expressed the highest temperature of 28.0 ± 0.05. The lowest mean salinity of 33.2 ± 0.5 was observed in control where as a highest salinity of 34.0 ± 0.2 was observed in pH 6. TA decreased with decreasing pH. The pH 6 expressed lowest alkalinity level of 2725.1 ± 0.05 µmol/kg whereas control had a highest level of 2741.2 ± 0.05 µmol/kg. DO levels were constantly monitored and maintained at ambient levels. The oxygen level decreased with increasing CO2. Control chambers maintained an ambient dissolved oxygen levels of 5.6 ± 0.1 whereas the pH 6 had the lowest level 4.9 ± 0.1.
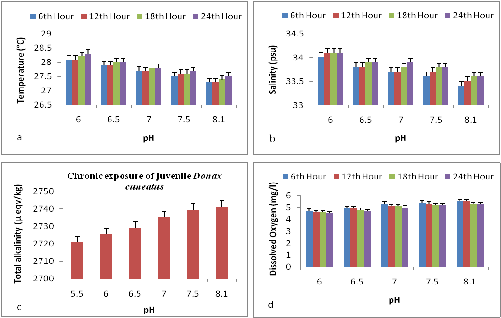
Figure 1 (a) Mean changes in the temperature of the microcosm chambers with respect to pH (b) Mean changes in the salinity of the microcosm chambers with respect to pH (c) Mean changes in the TA of the microcosm chambers with respect to pH (d) Mean changes in the DO levels of the microcosm chambers with respect to pH
For work in seawater, the pH(T) scale is the most commonly used scale and the recommended scale for monitoring activities .22 An important advantage in the use of this scale is that problems associated with the uncertainties in the stability constants for hydro fluorides are avoided, and the preparation of appropriate buffer solutions is simplified. Hence pH(T) scale was used in the present study to measure, monitor and maintain pH in the microcosm chambers. As per ICES report temperature plays a crucial role in the seasonal variation of pH. In the present study temperature in the microcosm chambers were closely monitored.
Hauton et al.23 in his microcosm study on Gammarus locusta reported a change of 0.1-0.2° C in temperature in the two selected nominal pH (7.8 and 7.6) compared across the control. In the present study mean changes of 0.2-0.6° C was observed in the nominal pH when compared across control. Nevertheless these changes may increase in a mesocosm environment. Hauton also reported 0.2-0.4 psu changes in salinity in his microcosm chambers where as in the present study mean changes of 0.2-0.6 psu where observed in the nominal pH when compared across control. These changes may vary in the mesocosm environment owing to the extensive volume of water and other environmental factors. The dissolved oxygen levels remained optimum throughout the experimental period with mean changes of 0.1-0.5 mg/l compared to the control. Since the water in the chambers was completely changed for every twenty four hours the change in the dissolved oxygen was minimum and remained optimum.
Mortality rate
Significant rate of mortality was observed in pH 5.5, 6.0 and 6.5 after 15 days of exposure where as no mortality was observed in pH 7.0, 7.5 and control. The effect of Hypercapnia on the percentage mortality of the exposed animals is summarized in Figure 2. Apart from the sub lethal effects of induced hypercapnia, the survival rate of the exposed animals also depends upon the microcosm environment. The physicochemical parameters of the microcosm chambers varied significantly (p<0.05) with varying pH regimes thereby indicating an inter relationship between pCO2 and seawater variables.
Biomarker analysis
Apart from physicochemical parameters most of the study to date focuses on the changes in calcification rate rather than on the physiological processes that induces these changes.24 There are many physiological processes that is affected by ocean acidification which leads to metabolic suppression, oxidative stress, apoptosis and symbiont loss.25-27 By measuring these physiological processes, any discrepancies observed in rate of calcification with respect to ocean acidification can be unraveled. Hence in the present study ocean acidification induced physiological changes observed in marine bivalve Donax cuneatus at the phenotype level using sensitive biomarkers.
Among the biological tools recommended for marine pollution monitoring, biomarkers have been successfully incorporated in the assessment of the quality of the coastal environment.28,29 Biomarkers provide early warnings on the sublethal physiological impacts .30 Aquatic organisms, especially marine bivalves, exhibit a variety of changes in enzymatic antioxidant defenses after exposure to pollutants with oxidative potential31 In the present study we have estimated the activity of AChE an important enzyme that hydrolyzes the neurotransmitter acetylcholine, the antioxidant enzyme CAT and LPO indicators of oxidative stress, the activity of RG and GST, an enzyme of the phase II of biotransformation that catalyzes the conjugation of RG to cellular components damaged by reactive oxygen species attack, leading to their detoxication process, to evaluate their utility as biological tools for ocean acidification monitoring in the Gulf of Mannar.
Total tissue protein: Measurement of the above said biomarkers are greatly dependent on the levels of total tissue protein levels. The total protein concentration in the tissues of the exposed animals decreased gradually with decreasing pH. The animals exposed to the lowest pH of 6 expressed a value of 0.5891g of protein/g tissue compared to the control which exhibited the value of 2.4174 g of protein/g tissue (Figure 3a). Significant changes (p<0.05) were observed between the animals exposed to the control seawater and the animals exposed to the nominal pH regimes.
Acetylcholine esterase activity: In the present study the animals exposed to all nominal pHs displayed an increase in activity of all antioxidant enzymes except AchE, which displayed decrease in activity. As per Podolska & Napierska.32 AchE tends to increase in animals existing in cleaner environment hence the decrease in AchE levels in the present study may be attributed to OA induced oxidative stress. The AchE enzyme activity decreased with decreasing pH levels. The activity decreased from 0.0024 µmols min-1 mg-1 of total protein to 0.0001 µmols min-1 mg-1 of total protein in pH 6 after 15 days of exposure (Figure 3b).
Catalase activity: An increase in the levels of CAT and RG has been reported in sea anemones exposed to copper chloride which was further intensified at low.33-35 Similarly in the present study increase in CAT, RG and GST levels at decreased pH were observed which might be further intensified by environmental toxicants. Stress induced increase in the enzyme activity was observed by CAT assay. The enzyme activity increased from 0.010 min-1 mg-1 protein to 0.038 min-1 mg-1 protein in the lowest pH of 6 after 15 days of exposure. A significant increase (p<0.05) in the enzyme activity was observed (Figure 3c). Significant differences were observed between the control and hypercapnia treated animals.
Glutathione - s - transferase activity: The GST enzyme activity increased with decreasing pH. Control exhibited an activity of 0.0025 µmol min-1 mg-1 of total protein when compared to pH 6 which expressed an activity of 0.0264 µmol min-1 mg-1 of total protein (Figure 3d).
Lipid peroxidation: Pimentel et al.36 reported increased levels of lipid peroxidation (based on MDA levels) under high temperature and pCO2. Correspondingly lipid peroxidation (also based on MDA levels) increased at low pH and high pCO2 in the present study. The level of MDA increased from 0.251nmol/mg (Control) to 0.858nmol/mg in pH 6 after 15 days of exposure. The enzyme activity increased significantly (p><0.05) with decreasing pH (Figure 3e).
Reduced glutathione activity: The level of RG gradually increased with decreasing pH after 15 days of exposure. Significant changes (p<0.05) were observed in the enzyme activity due to induced hypercapnia. The RG levels increased from 0.20 μ mol/g (Control) to 0.36 μ mol/g in the pH 6 after fifteen days of exposure (Figure 3f).
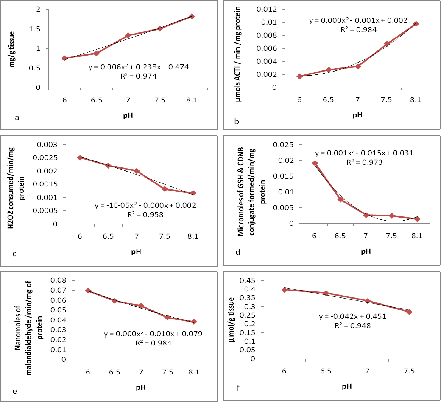
Figure 3 (a) Impacts of Hypercapnia on total protein concentration. (b) Impacts of hypercapnia on AchE activity. (c) Impacts of hypercapnia on CAT activity. (d) Impacts of hypercapnia on GST activity. (e) Impacts of hypercapnia on lipid peroxidation (f) Impacts of hypercapnia on RG activity.
Cossu et al.37 reports that under stress the antioxidant system of the animals may either be activated or inhibited. The activation and inhibition are two contradictory responses that are dependent on the duration and intensity of the stressors as well as on the sensitivity of the animals exposed. Activation indicates the adaptation of the animals to the stressor where as inhibition indicates later stage of oxidative stresses.38
Two ways ANOVA revealed that all the seawater variables showed highly significant change across pH and time except salinity which exhibited marginally significant change across time (Table 1). The one way ANOVA for biomarkers had a significant result (Table 2).
|
S. No |
Parameters |
Variation Across pH |
Variation Across Time |
||
|
|
|
F |
P |
F |
P |
|
1 |
Temperature |
327 |
0.000 |
36.66 |
0.000 |
|
2 |
Salinity |
204.5 |
0.000 |
18.7 |
0.000 |
|
3 |
DO |
181.1 |
0.000 |
25 |
0.000 |
|
4 |
TA |
356.6 |
0.000 |
- |
- |
Table 1 Two Way ANOVA on the effects of hypercapnia on seawater variables across pH and time
|
Source of Variation |
SS |
df |
MS |
F |
P-value |
F crit |
|
Between Groups |
0.368255 |
4 |
0.092064 |
70.7414 |
1.63E-11 |
2.866081 |
|
Within Groups |
0.026028 |
20 |
0.001301 |
|||
|
Total |
0.394283 |
24 |
Table 2 One way ANOVA on the effects of hypercapnia on biomarkers
The R2 value for each biomarker along with their trends has been represented in Table 3. Regression analysis of all the biomarker enzymes in the present study displayed value nearer to one there by having a perfect fit to their respective trend lines.
|
S. No |
Biomarker |
Regression |
Trend |
|
1 |
Protein |
0.9746 |
Logarithmic |
|
2 |
Acetylcholine Esterase |
0.9844 |
Polynomial |
|
3 |
Catalase |
0.9588 |
Polynomial |
|
4 |
Lipid Peroxidase |
0.9849 |
Linear |
|
5 |
Glutathione S Transferase |
0.973 |
Logarithmic |
|
6 |
Reduced Gltathione |
0.948 |
Linear |
Table 3 Regression analysis of the effects of hypercapnia on biomarkers and protein
Extensive variation has been observed so far in the response of inter specific species towards OA. In order to discriminate and understand these responses and their impacts there is a strong need to imply a sensible and sensitive toxicology testing to monitor the impacts of ocean acidification. Such stress response evaluation is valuable to monitor OA impacts in the provinces vulnerable to industrial contaminant, since proficient testing of impacts induced by toxicants that are acid dependent can be monitored effectively. Biomarkers used in the present study has proven to be one such stress monitoring tool used in effective monitoring of OA as well as environmental contaminants. From the present study it is concluded that juvenile marine bivalves Donax cuneatus responds negatively with respect to ocean acidification. Donax cuneatus are highly sensitive to lower pH and endure extreme oxidative stress by OA alone as evident from the control group. However these impacts can be further intensified by other toxicants prevalent in the environment hence further intensive and cumulative studies are required to examine the combined impacts of ocean acidification and environmental toxicants.
The first author is thankful to the University Grants Commission for providing Basic Science Research fellowship and DST PURSE for the consumable support for completing the study.
None.

©2016 Priya, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.