Journal of
eISSN: 2378-3184


Research Article Volume 4 Issue 2
Mindanao State University at Naawan-School of Marine Fisheries and Technology, USA
Correspondence: Edwin Estares Dumalagan, Mindanao State University at Naawan-School of Marine Fisheries and Technology, 9023 Naawan, Philippines, USA
Received: November 12, 2015 | Published: March 9, 2016
Citation: Dumalagan EE (2016) Biomass and Phytal Animals of Floating. J Aquac Mar Biol 4(2): 00075. DOI: 10.15406/jamb.2016.04.00075
The biomass and phytal animals of floating Sargassum were studied in two stations Lugait (S1) and Naawan (S2), Misamis Oriental on August 14, 2008. Two traps were constructed in S1 and S2 to collect the floating Sargassum and to remove the phytal animals by using glass slide, forceps and brushing technique. Wet and dry weight biomasses of the Sargassum thalli were determined. Physico-chemical parameters such as salinity, temperature and depth were noted in both S1 and S2. The results showed that five groups of phytal animals were identified: foraminifera’s (28 ind kg-1 Sargassum thalli), shrimp post larvae (6 ind kg-1 Sargassum thalli), sponges (4 ind kg-1 Sargassum thalli), gastropods (3 ind kg-1 Sargassum thalli) and polychaetes (2 ind kg-1 Sargassum thalli) in S1. Foraminifera’s (44 ind kg-1 Sargassum thalli), shrimp post larvae (3 ind kg-1 Sargassum thalli), sponges (2 ind kg-1 Sargassum thalli), gastropods (1 ind kg-1 Sargassum thalli) and polychaetes (1 ind kg-1 Sargassum thalli) in S2. Relative abundance of the phytal animals yielded foraminifera’s as the highest range from 67% (S1) to 90% (S2).
This was followed by shrimp post larvae with a range from 5% (S2) to 13% (S1). The remaining phytal animals were at low relative abundance with ranges from 3% (S2) to 9% (S1) for sponges; from 1% (S2) to 6% (S1) for gastropods; and from 1% (S2) to 5% (S1) for polychaetes. The WW biomass of Sargassum thalli minus the phytal animals were 4.75 WW kg-1 (S1) and 4.25 WW kg-1 (S2). S1 had high evenness (0.6627) accounting to high diversity (0.4632) with low dominance (0.4816). Whereas in S2 evenness was low (0.2747), with high dominance (0.8162) characterizing low diversity (0.1920) in the area. The importance of the phytal animals on floating Sargassum in the marine ecosystem must be considered in the formulation of pelagic environment conservation measures and policies.
Keywords:Biomass, Sargassum spp, Floating, Phytal animals
Rationale
The Philippines is an archipelagic nation where it is composed of 7,105 islands and islets. These islands and islets are surrounded by coastlines which have a general total area of 18,000 km2. These coastal areas are distinct which have a particular combination of different environmental factors that create localized effects that affect the community composition of different ecosystem. In such communities different species survive the environment they are accustomed to inhabit, and sometimes are subjected to habitat fragmentation which are usually caused by human alteration,1 and this includes Sargassum beds which house unique and unusual animals.2
In South Africa, Sargassum has high productive capacity during the autumn and winter months and only average in the warmer months3 and have attained a maximum sustainable yield (MSY) that is estimated to be 100,000 metric tons wet weight per year.4 Aside from that, floating Sargassum and Sargassum beds are one of the diverse ecosystems in the marine environment, where it is home to some species in the sea and it is a breeding, spawning, hiding and eating ground for young fishes and sea turtles. In fact in Mississippi, a species of juvenile fishes, jacks and trigger fish are found beneath these beds.5 The Sargassum also house the suitable attachment for epiphytes that carry with them while floating.6 The importance of this floating Sargassum have different significant in its destination, like for landward, it helps the beaches and dunes as a natural barriers against storm flooding and beach erosion. The organism in the thalli can be also a fertilizer. In seaward, young tuna, marlin, game fish and jellyfish swim with it and sometimes it can be a food for them.5
However this seaweed is in the state of threat due to its excessive harvest for feeds, fertilizer and alginate. Moreover, live Sargasssum reflect the result of anthropogenic activity, like the siltation inflow from upland, including various wastes from factories that degrade this Sargassum. Ecological importance of the epiphytes attached to Sargassum is recognized. Thus this study will provide the awareness on how to protect and prevent the Sargassum against destructions which contains the biota load that is ecologically important.
This study is conceptualized based on the following hypothesis:
Over the period of years, there is a poor understanding regarding the Sargassum spp. The knowledge of these species is essential particularly the floating Sargassum to further emphasize in fisheries production.
Objective of the study
General objective: The study generically aims to examine and compare the biota animals an biomass of floating Sargassum spp. in Lugait and Naawan, Misamis Oriental.
Specific objective: To determine and compare the presence and abundance of phytal animals in floating Sargassum spp. in the collected thalli in the two selected areas. To determine and compare the biomass of floating Sargassum spp. in the two selected areas.
Significance of the study: The result of the study shall provide baseline information on the current condition of floating Sargassum in terms of their biota and biomass in Lugait and Naawan, Misamis Oriental. Furthermore, the result shall provide environmental advice on how to manage Sargassum beds to support fisheries production.
Scope and limitation of the study: The study was focus only on the macrofaunal biota and the biomass of floating Sargassum spp. The phytal animals identified from Sargassum spp thalli were counted in which the biota was classified according to their major groups due to the unavailability of related resources.
Definition of terms
Biomass: Biomass is the total weight of all organisms in a given area or volume.7
Biota: Biota refers to all the living organisms occurring within a certain area or region. It is also called epiphytes that are usually present on leaf surface of submerged aquatic macrophytes. The term epiphytes refer to heterotrophic and autotrophic organisms composing diverse communities.8
Floating: Flaoting Sargassum is free floating seaweed found throughout the blue waters of the south atlantic from extensive commercial harvest.4
Macrofauna: Macrofauna refers to animals whose shortest dimension is greater than or equal to 0.5mm.9
Phytal animals: A Phytal animal refers to animals that inhabit the thalli of Sargassum spp.10,11
Salinity: Salinity refers to number of grams of dissolved salts in 1000g of sea water.9
Sargassum spp is a type of algae which has a small air bladder called vesicles that contains carbon dioxide which makes them to float.12 Even if the thalli detach from its holdfast and it floats, it is still capable to survive.13 Thus, it is one source of organic matter in the pelagic community. Floating Sargassum spp is a floatsam.14 The significance of this floating Sargassum has relevance to its destinations. For landward, Sargssum helps to build strong sand dunes against storm flooding and beach erosions.12 For seaward, it provides a habitat for pelagic organisms, thereby forming large mats14 where organisms swim with it and can be a potential step for fish trading.15 A particular fish,16 which mimics the colour of weed, was discovered from the drifting Sargassum weed in India.17
Sargassum is one of the most diverse ecosystems, because it is considered as home for other organisms in the sea.4 It houses the epiphytes carried with them while floating.6 The biota of Sargassum largely composed of diatoms which have a >93% of the total population.18 He also mentioned that during the development of this biota community, the density and the biomass of the attached organisms’ increases on the older part thallus compared to the younger part. Many organisms were more abundant on plant part taken closer to the water surface than on plant part taken from deeper water. It is said that organisms living in this algae provide habitat complexity.19 The Sargassum and the epiphytes can benefit the flow of energy and the cycle of matter.20
Sargassum has different seasonality in biomass production. It was stated that S. baccularia attained high biomass during January, while S. binderi attained high biomass during April.7 Both species are highly correlated in the length of the thallus with rainfall.3
Aside from its habitat importance, Sargassum spp is utilized as feeds and fertilizers.7 It is good source of alginate which is used as thickener, emulsifier, stabilizer.3 It is a multifunctional food for maintaining health because it is rich in minerals, vitamins and dietary fiber. In Japan, it is used as savory food.6 Sargassum spp. biomass has been used for the removal of metal waste water since it is a biosorbent.21 According to Karthikeyan et al.22 this biosorption technology uses a much cheaper material like the Sargassum spp. Sargassum spp algae contains mainly the polysaccharide alginate, usually calcium and sodium alginate, that is said to have the high potentials for the accumulation of heavy dissolved metals in the different water system.22 The biosorbent with high adsorption process is generally rapid and it is suitable for the great extraction of metal ions from large volumes of water that is already accumulated.23 A particular Sargassum species, S. muticum which is considered an invasive species is said to be the most accurate to use in the binding analysis with non great deal thermodynamically consistent absorption because of its high content of alginate.24 In the light of above mentioned studies, the floating Sargassum spp and the biota composition will be investigated so as to understand its implication to the environment.
Study area
The study was conducted in the two stations: Poblacion, Lugait, Misamis (S1) Oriental and in Poblacion, Naawan, MIsamis Oriental (S2). The stations were established by using the GPS (Global Positioning System) (Figure 1) (Table 1).
|
|
Lugait |
Naawan |
||
|
North |
East |
North |
East |
|
|
Trap 1 |
08⁰20' 40.1" |
124⁰15' 27.6" |
08⁰25' 52.4" |
124⁰17' 12.0" |
|
Trap 2 |
08⁰20' 41.3" |
124⁰15' 51.1" |
08⁰25' 51.1" |
124⁰17' 10.7" |
Table 1 Geographical location of the traps in Lugait (S1) and Naawan (S2), Misamis Oriental
Description of the Sargassum spp.
Sargassum spp, is a member of brown algae in Class Phaeophyta.5 Its life cycle consist only of large pseudo-perennial plant,13 which reproduced by means of receptacles born in the secondary branches and contains both oogonia and antheridia.25 The distinct characteristics that differ them to other seaweed, is the small air bladder called vesicles that contains carbon dioxide which makes them to float12 and can make them stand upright in the water while they are still attached in the bottom (Figure 2).Sampling time and frequency
Sampling was done on August 14, 2008 simultaneously for 24 hr in S1 and S2 to obtain the same weather condition that controls the floating Sargassum spp. 3.4. Field activities
Collection of floating Sargassum spp.
Two traps (Figure 3) were installed in every station.26 The trap was 3m x 3 m in length and width. The net stretched up to 3 m with a mesh size of 1.2 cm. It was made up of bamboo poles and plastic nylon net. The trap was installed in the Sargassum bed by digging two - three meter distant holes to erect the bamboo poles. The net was tied in both poles and the tail part of the net was anchored for stretching.
The trap in Lugait was installed in the morning of August 13, 2008 and this was followed by the installation of the trap in Naawan in the afternoon. Collection of Sargassum in the traps at 1 kg trap-1 was done for Sargassum spp biomass determination.
Biota and biomass determination
The trapped Sargassum thalli at 1 kg trap-1 were placed in pre-labelled sealable bottles which contained 5% buffered formalin to preserve the phytal organisms and the Sargassum for biota and biomass determination, respectively.
Laboratory activities
Floating Sargassum: After the sampling, the collected samples was then weighed using the Ohaus triple beam balance and prepared for phytal animals and biomass determination. After the removal of the phytal animals, the thalli were processed for wet weight (WW) determination.
Biota determination: At MSU Naawan laboratory, the collected samples (Figure 4) were washed inside a sieve with mesh size of 20 µm (Figure 5). The removal of the phytal animals was done by using the brushing technique27 (Figure 6). Phytal animals that were not removed from the thalli were scraped by using a glass slide (Figure 7)28 or by using forceps (Figure 8 & 9).14 All collected organisms per 1 kg of Sargassum were placed in the pre-labelled bottle with 5% buffered formalin. Photomicrographs of the removed phytal animals were taken by using a Kodak digital camera. The biota per 1 kg of Sargassum was observed with the aid of a Swift light microscope and the species were counted and categorized according to major groups. The Sargassum spp were dried and weighed to constant weight for biomass determination which was expressed in DW biomass15
Physical parameters
Aside from the WW and DW determination of Sargassum thalli other parameters determined were tides, depth, temperature and salinity. Tide cycle was obtained from a calendar which was used as the basis for the stretching of the trap. Water samples were collected for salinity analysis by using the ATAGO refract meter (Figure 10). Temperature readings were obtained by using a mercury filled thermometer. The depth was obtained by using a calibrated post of each trap.
Data management
Diversity indices: Species diversity was determined using the following formula by Pielou.29
Index of dominance:
C=Σ(ni/N)2
Where:
ni is the importance value of each taxa
N is the total importance value of all taxa
Shannon-Weiver index of general diversity (H’):
H’ =–Σ(ni/N) log
Where:
ni is the total importance value of each taxa
N is the total number of individuals identified per unit area
Index of evenness(e):
E= (H’/logs)
Where:
H’ is the Shannon-Weiver index
S is the total number of species
Index of similarity
S=2C/A+B
Where:
A is the number of species in sample A
B is the number of species in sample B
C is the number of species common to both A and B
Relative abundance
The relative abundance of biota and biomass was calculated using the formula by Odum.30
Relative abundance (%) =Number of individualsTotal number of individuals
Abundance of phytal animals in floating Sargassum spp.
In Lugait, there were 5 groups of phytal animals identified, namely: shrimp post larvae, sponges, foraminifera’s, gastropods and polychaetes. Among the 5 groups, the foraminifera’s showed the greatest number of individuals which had a total mean population density of 28 ind 1 kg-1 Sargassum spp thalli followed by shrimp post larvae (6 ind kg-1 Sargassum thalli), sponges (4 ind kg-1 Sargassum thalli), gastropod (3 ind kg-1 Sargassum thalli) and polychaetes (2 ind kg-1 Sargassum thalli).
In Naawan, 5 groups of phytal animals were observed with foraminifera’s which had a population density of 44 ind kg-1 Sargassum thalli as the most abundant. Shrimp post larvae (3 ind kg-1 Sargassum thalli), sponges (2 ind kg-1 Sargassum thalli), polychaetes (1 ind kg-1 Sargassum thalli) and gastropod (1 ind kg-1 Sargassum thalli) were present in low population densities (Table 2) (Appendices).
|
Mean densities( ind kg-1 Sargassum thalli) |
||||
|
Phytal animals |
Lugait |
% |
Naawan |
% |
|
Foraminiferans |
28 |
65 |
44 |
86 |
|
Shrimp post larvae |
6 |
14 |
3 |
6 |
|
Sponges |
4 |
9 |
2 |
4 |
|
Gastropods |
3 |
7 |
1 |
2 |
|
Polychaetes |
2 |
5 |
1 |
2 |
Table 2 Phytal animals identified and their mean densities in S1 (Lugait, Misamis Oriental) and S2 (Naawan, Misamis Oriental) on August 14, 2008
Biomass of floating Sargassum spp.
The total WW of floating Sargassum trapped in S1 and S2 yielded to 4.75 kg and 4.25 kg, respectively. In Lugait, 3.25 kg (First trap) and 1.5 kg (second trap) were collected which totalled to 4.75 kg WW. In Naawan, the first trap had 3.5 kg and the second trap had 0.75 kg which totalled to 4.25 kg (Table 3). The differences in the WW of Sargassum spp could be attributed to the position of the trap wherein the trap was installed facing perpendicular to the current. The opening of the trap was not intended to trap floating Sargassum spp which drift with the current but to trap floating Sargassum during flooding high tide and low tide. It was observed that the first trap in both stations got the highest value of captured biomass; this was observed because the first trap was located in accordance to the direction of the current. The current was moving towards northeast or southeast which was relatively going to the east based on the drift bottle technique used in the study.
|
1 Trap |
2 Trap |
Total |
||||
|
Station |
WW |
DW |
WW |
DW |
WW |
DW |
|
Lugait |
3.25 |
0.2128 |
1.5 |
0.4522 |
4.75 |
0.665 |
|
Naawan |
3.5 |
0.1301 |
0.75 |
0.5929 |
4.25 |
0.723 |
Table 3 WW and DW biomass of Sargassum spp per trap in kg in Lugait and Naawan, Misamis Oriental on August 14, 2008
The DW biomass of Sargassum in Lugait was 0.665, that is, about 86% of water was lost from the original weight of 4.75 kg whereas 83% of water was lost in the DW of Sargassum in Naawan from 4.25 kg WW to 0.723 kg DW.
Relative abundance of phytal animals in floating Sargassum spp in Lugait and Naawan, Misamis Oriental on August 14, 2008
Result of the relative abundance of biota S1 (Lugait) and S2 (Naawan), showed that foraminifera’s (Figure 11) were the most abundant in both stations. Among the 5 groups of phytal animals identified, shrimp post larvae (13%) (Figure 12), sponges (9%), gastropods (6%) (Figure 13) and polychaetes (1%) (Figure 14) in Naawan were higher in abundance in Lugait station, compared to shrimp post larvae (5%), sponges (3%), gastropods (1%) and polychaetes (1%) in Naawan station. However, foraminifera’s (67%) in Lugait station was lower in comparison to the foraminifera’s (90%) in Naawan Station (Figure 15 & 16). The dominance of biota of certain phytal animals in floating Sargassum spp indicated that floating Sargassum spp is the habitat of some pelagic and benthic marine organisms.2
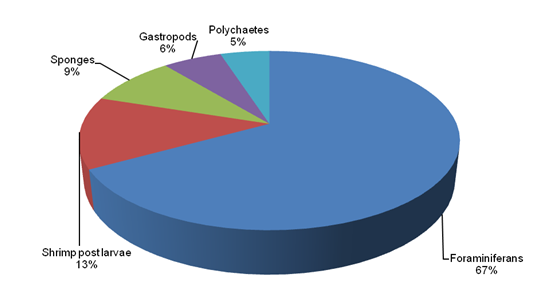
Figure 15 Relative abundance of phytal animal’s infloating Sargassum spp in Lugait, Misamis Oriental.
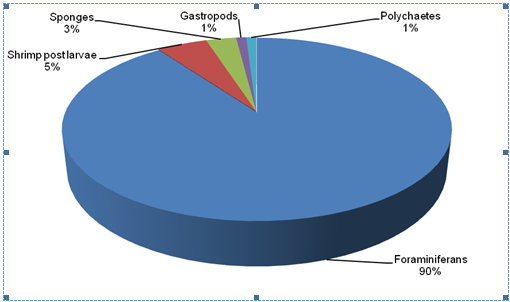
Figure 16 Relative abundance of phytal animals in floating Sargassum spp in Naawan, Misamis Oriental.
Relative Abundance of WW biomass of floating Sargassum spp in Lugait and Naawan, Misamis Oriental
The relative abundance of WW biomass of floating Sargassum spp showed that the first trap got the highest trapped biomass (82%) than second trap (18%) in Naawan. While in Lugait, the first trap (68%) obtained the highest value in comparison to the second trap (32%). However, the first trap in Lugait had lower WW biomass in comparison to the first trap in Naawan, and the second trap in Lugait had higher WW biomass in comparison to the second trap in Naawan (Figures 17 & 18). This indicates that there was slight difference of drifted Sargassum spp in the two stations irregardless of the location and direction of the traps installed.
Diversity Indices
Diversity indices showed that in Lugait, the index of dominance (C) was 0.4816, the Shannon-Weiver index of general diversity (H’) was 0.4632 and the index of Evenness (e) was 0.6627. In Naawan, the index of dominance (C) was 0.8162, the Shannon-Weiver index of general diversity (H’) was 0.1920 and index of evenness was 0.2747. These results indicate that diversity was high in Lugait with high evenness and low dominance. In Naawan there was dominance of one species so the diversity was lower in comparison to that of Lugait (Table 3). The index of similarity was 1 which could be attributed to the presence of similar species identified in both stations.
Bio-physico-chemical parameters
Results of the environmental parameters in Lugait and Naawan, showed that 29.60C reading of water temperature in Lugait was higher in comparison to Naawan which was 28.90C. Water salinity was 32 0/00 for Naawan which was higher than that of Lugait which was 29 0/00. The Lugait estuary was located in the western portion of the study site while the Naawan estuary was located in eastern portion of the study site. Every time fresh water flowed in the open water, this was carried by the current which moved relatively to the east, and the Lugait study site was affected with this mixture of water than the study site in Naawan which was not affected by this water flow system. Thus, both stations had different water salinity. Water depths gathered were 1.2 m in Lugait and 1 m in Naawan (Table 4 & 5).
|
Station |
C |
H' |
E |
|
Lugait |
0.4816 |
0.4632 |
0.6627 |
|
Naawan |
0.8162 |
0.192 |
0.2747 |
Table 4 Diversity of phytal animals collected from floating Sargassum thalli in Lugait and Naawan, Misamis Oriental
|
Station |
Temperature (0C) |
Salinity (0/00) |
Depth (m) |
|
Lugait |
29.6 |
29 |
1.2 |
|
Naawan |
28.9 |
32 |
1 |
Table 5 Physico-chemical parameters determined in Lugait and Naawan, Misamis Oriental
Biomass and phytal animals of floating Sargassum in Lugait (S1) and Naawan (S2) in the province of Misamis Oriental were studied simultaneously last August 14, 2008 with the use of traps to capture floating Sargassum. Glass slide, forceps and brushing technique were used to remove the attached organisms on the Sargassum spp thalli. Floating Sargassum collected in each trap was analyzed for WW and DW biomass.
There were 5 groups of phytal animals identified in the two stations, namely: Shrimp post larvae, sponges, foraminifera’s, gastropods and polychaetes. The result showed that the population densities of foraminifera’s were high in both areas. In Lugait, the shrimp post larvae, sponges, gastropods and polychaetes were high in population densities in comparison to Naawan. However, the foraminifera’s in Lugait had lower population densities in comparison to Naawan. The index of dominance (C) was 0.4816, Shannon-Weiver index of general diversity (H’) was 0.4632 and the index of evenness (e) was 0.6627 in Lugait. In Naawan, the index of general diversity (H’) was 0.1920; index of dominance (C) was 0.8162, and the index of evenness was 0.2747. This result suggests that diversity was high in Lugait in comparison to Naawan. In Naawan, there was greater dominance of foraminifera’s in comparison to Lugait, thus the low diversity in the area. The index of similarity of the phytal animals in both stations was 1 which indicated that both S1 and S2 had high similarity in species composition.
The foraminifera’s were the most abundant in both areas. Shrimp post larvae 13%, sponges 9%, gastropods 6% and polychaetes 5% were of high percentages in Lugait in comparison to Naawan which had relative abundance values of 5% for shrimp post larvae, 3% for sponges, 1% for gastropods and 1% for polychaetes. However, foraminifera’s with 67% in Lugait was lower in relative abundance in comparison to that of Naawan which was 90%.
The WW biomass of floating Sargassum spp. showed that, in Naawan, the first trap had 82% which was higher in comparison to the second trap with 18% only. In Lugait, the first trap got 68% which was also a higher value of collected biomass in comparison to that of the second trap which had only 32%. Environmental parameters gathered showed 290C for water temperature, 29 0/00 for water salinity and 1.2 m for water depth in Lugait. In Naawan, 28.90C was the water temperature, 32 0/00 was the salinity and 1 m was the water depth.
None.
None.

©2016 Dumalagan. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.