Journal of
eISSN: 2378-3184


Research Article Volume 4 Issue 1
Department of Business and Primary Industries, Melbourne Polytechnic, Australia
Correspondence: Sadiqul Awal, Bachelor of Agriculture & Technology degree program (Aquaculture), Department of Business and Primary Industries, Melbourne Polytechnic, Cnr Cooper St & Dalton Rd, Epping, Victoria 3076, Australia, Tel +61392691162
Received: December 20, 2015 | Published: February 23, 2016
Citation: Awal S, Christie A, Nieuwesteeg D (2016) Substrate Selectivity and Food Preference of the Caprellid Amphipod (Caprella Penantis); Evaluation of a Possible Aquaculture Resource for Marine Hatcheries. J Aquac Mar Biol 4(1): 00073. DOI: 10.15406/jamb.2016.04.00073
Marine aquaculture is growing at a significant rate, with an increasingly diverse range of fish being cultured. In order to maintain and enhance the growth and prosperity of the industry, additional feed sources are required for different fish species. Caprellid amphipods have been identified as a possible candidate for exploitation as an aquaculture live feed. Caprella penantisis a globally distributed caprellid species found in the littoral zones of many coastal habitats throughout the world. Like several other species, it has been acknowledged as one of the most suitable species for culture, due to its diverse range, and wide environmental tolerances. This paper examined the survivability of C. Penantis when reared on different substrate types in closed systems. Laboratory experiments used 20L carboys as culture vessels, with each being equipped with a particular culture substrate. The substrates that were tested in this experiment included natural substrates, artificial substrates and no substrate, and consisted of Sargassum spp., seaweed and rope, respectively. In addition, feeding habits, uptake and population growth performance on each of the different substrate types were observed, monitored and assessed over a sixteen-week trial period.
The use of artificial substrate produced the highest survivability and population growth, in addition to the highest feed uptake. Average survival for populations of the control, natural and artificial substrate groups were 31%, 14% and 82% respectively, suggesting that artificial substrate is the most suitable culture medium for this particular caprellid. The study found the culture of caprellids to be of relatively high labour intensity, particularly when compared with currently used live feeds; they are perhaps therefore best suited for use in small-scale mariculture systems that have been established for the production of high value species.
Marine aquaculture is developing at a rapid rate in response to the global increase in seafood consumption. Advances in the use and increases in the production of natural and live feeds are required to meet the nutritional requirements of larval fish and crustaceans,1 which underpin the burgeoning global mariculture industry. Such larval live feeds are required for development in a way that meets the goals of an industry driven towards sustainability and efficiency.
Marine aquaculture currently relies on a limited number of live feed organisms for larval fish culture, such as Artemia, rotifers and (to a much lesser extent), copepods.1 Standardised protocols for the culture of these organisms have been developed into efficient methods of production.2-6 These live feeds, however, present a limited dietary value that may not be suitable for all species being cultured.7 In addition, further enrichment is required to ensure proper development of marine species, though this can lead to excess dietary lipid concentrations and sub-optimal levels of some vitamins, minerals and amino acids.5 Many other macroinvertebrates have recently been studied as potential sources of live feeds for marine fish larvae. To satisfy the growing demand, research has taken steps to develop new innovative live feeds. For example, several studies have focused on sand worms and blood worms,8 bait worms, beach worms, quill worms,9 nematodes,10 and polychaete worms,11 as a potential food source for aquaculture hatcheries.
Caprellid amphipods, also known as skeleton shrimp, are small peracaridean crustaceans that inhabit a variety of substrata from the intertidal to subtidal zones,12-14 though their abundance decreases towards the poles.15 They are common members of epibiotic fouling communities.16-18 They are relatively sedentary,19 lacking a planktonic stage, and appear to be relatively poor swimmers, moving through the water in a manner that appears to be inefficient (pers. obs.), and which involves rapid ripples of the entire body, which is said to occur most commonly amongst juveniles when agonistic interactions20,21 occur. Caprellids therefore appear to swim only as a last resort, with typical instances occurring if they are disturbed and dragged up through the water column; caprellids will then be readily seen swimming in an apparent effort to try and locate a substrate and/or the benthos (pers. obs.). For the most part, caprellids move along a substrate with a movement that is not unlike a caterpillar or leach (pers. obs), grasping the substrata with their specialized hind pereopods and holding on alternately with hind pereopodsor their gnathopods as they inch their way along the substratum.20,21
Caprellids generally spend their entire lives attached to firm substrates, which can include items such as various species of macroalgae, seagrasses, hydroids, bryozoans, ascidians, and sponges.21-28 They are found to a lesser extent on soft-bottom benthic habitats.28,29 Many species appear to be relatively non-selective with respect to their substratum when presented with characteristic habitat types,25,30 although some may exhibit substratum preferences.31,32 Complex substrata appear to facilitate higher caprellid abundances through their larger surface areas, and possibly from the greater degree of protection from predators arising from such complex surfaces.27 Caprellids readily colonize artificial structures such as ropes, fish cages, netting, buoys, pontoons, and oil platforms33-38 (pers. obs). Indeed, caprellid populations may be much denser on artificial structures than on surrounding natural substrata,25,36 indicating that anthropomorphic structures could well be vital in the ecology of these amphipods.
Under appropriate conditions, caprellids can attain high biomass, particularly in environments with high organic content, such as around fish farms.1,39 Caprellids are important prey items for many coastal fish species such as red fingers (Cheilodactylus fasciatus), kelp perch (Brachyistius frenatus) and surfperch (Embiotica Lateralis)1,20,21 and form an important trophic link between primary producers and higher trophic levels.1 It is extremely likely that the actual number of different marine finfish species that would be suited to a diet of caprellid amphipods would be much higher than the three species highlighted previously, as will be discussed in more detail later.
Caprellid amphipods have been the source of some studies demonstrating their suitability for exploitation as a potential marine fish feed. Guerra-Garcia et al.40 found that caprellids have high levels of desirable polyunsaturated fatty acids including EPA (Eicosapentoic acid), DHA (Docosahexanoic acid) and ARA (arachadonic acid), as well as many other major fatty acids, though their overall nutritional value is not well known.1 It has been demonstrated that some fish species, including some commercially important food and ornamental species (including Chinook salmon, Oncorhynchus tshawytscha, yellow tail, Seriola quinqueradiata, and various species of Sygnathids such as seahorses and pipefish) rely on caprellids for sustenance, particularly in the early life stages(from larvae to juveniles). Some species of fish that attain relatively small maximum sizes also rely on caprellids when moving towards the adult stages.1
As mentioned previously, anthropomorphic features may be colonised to relatively high densities by caprellids; a good example is sea cages, whose materials have been found to attract high densities of caprellids, which in turn may become a significant (or sometimes, relatively minor) feature of the diet of the finfish that are farmed in such cages.41,42 Caprellids from cage netting were found to be the most important wild food organism consumed by caged Atlantic salmon in British Columbia according to studies by Hay et al.42 who estimated that as much as 3.3% of total salmon gut contents by wet weight came from these amphipods.
This study analysed survivability of a local species of caprellid Caprella penantis (Figure 1) when cultured on different substrates. Caprella penantis Leach, 1814, is a Caprellid amphipod characterised by its well-developed head and rostrum, and elongate gill structures on pereonites 3 and 4, separating it from morphologically similar species of C. danilevskii, C equilibria and C. Dilatata.43 Caprella penantisis regarded as one of the most difficult species to classify, and has been recorded under many different species and subspecies. Originally it was believed to be a complex of different species, due to its morphological variation between populations in different locations, however genetic studies found that it was of a single species, with morphological differences occurring as a result of environmental factors such as wave exposure or turbulence, in which those found in more exposed areas develop a more robust body.44 C. Penantis is a globally distributed species,44 found on red and brown algae, seagrasses, hydroids, bryozoans, sponges and attached to echinoderms.43 Caprellids spend most of their time in an upright-parallel posture, leading some to liken them to the praying mantis (Figure 2). It has been suggested that feeding is conducted primarily by scraping the substratum, which could be considered an advantage in exposed environments.45
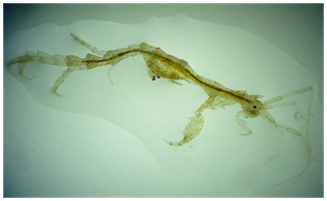
Figure 1 A Caprellid amphipod, Caprella penantis, female viewed under a dissection microscope (lateral view). (Photograph: Damien Nieuwesteeg, Melbourne Polytechnic).

Figure 2 A caprellid in the characteristic “praying mantis”-style upright posture (left) (Photograph: Sheree Marris), and many individual caprellids covering the glove of a snorkeler after an inadvertent brush against macroalgae (right) (Photograph: Andrew Christie, Melbourne Polytechnic).
As mentioned previously, Caprellids spend their whole life attached to a substratum surface.12 The ability to cling to the substrate is without doubt a well-adapted survival method,46 though it presents a challenge for aquaculture, since if caprellids cannot be encouraged to let go of the substrate, it may be relatively difficult to offer them up as a live feed type to various fish species. Studies have so far suggested the use of a substrate that can be placed directly into the finfish tanks, so that the caprellids can then be “picked off” and consumed by the cultured finfish species1 at their leisure.
In the present study, the survivability of populations cultured on natural substrates (Sargassum spp.) and artificial substrate (rope) was observed, with added emphasis on feeding habits and condition analysis, in order to determine whether the use of an artificial substrate could provide both a suitable culture medium and feeding mechanism. This study aimed to determine the suitability of caprellids for aquaculture, relating to the substrate available, and both its effect on survivability and suitability for further use as a feeding tool. Once a suitable substrate was determined, the study further compared the growth of animals when offered one of three microalgae species, namely Dunaliella tertiolecta, Cylindrotheca fusiformis and Phaeodactylum tricornutum.
Specimens of Caprella penantis were collected from natural substrates in Port Phillip Bay, Victoria. Samples were obtained by snorkelling at depths from one to five metres. Caprellids were taken with samples of the natural substrate (Sargassum spp.) and placed in watertight containers for transport. A sufficient volume of water was collected from the same site for use throughout the experiment. The collection site, the Blairgowrie Yacht Squadron pier, was an ideal site for collection, with the anthropomorphic structures and adjacent rocky reefs and seagrass meadows providing plenty of habitat for biofouling communities. Collections were conducted on various August (winter) mornings, when caprellid population numbers are found to be somewhat lower than summer months.18
Once collected, populations were transported in insulated containers to the Live Feeds and Algology Laboratories at Melbourne Polytechnic’s Epping campus. Caprellids were subject to one of three substrate types, being natural substrate (Sargassum spp.) and artificial substrate (rope), with a trial that had no substrate acting as the control group. A total of nine carboys were arranged in three by three formation, with each carboy receiving a constant supply of aeration via a side channel blower, tubing for the reticulation of air, and an air stone for the distribution of dissolved oxygen throughout the culture structure. Prior to the carboys being filled, seawater was filtered down to sixty microns through mesh screens.
Two experimental trials were conducted: trial 1 consisted of determining the affinity of caprellids for various types of substrate under simulated hatchery conditions, while trial 2 consisted of determination of food preferences, as evidenced by population growth performance, again under simulated hatchery conditions. The experimental diets in trial 2 consisted of the three types of microalgae mentioned previously (D. tertiolecta, C. fusiformis and P. tricornutum) from pure cultures that featured no additional algal species.
Natural substrates were provided in the form of Sargassum (approximately 200mm long), the same substrate from which the animals were collected. Sargassum (a branched macroalgal species with a three-dimensional shape consisting of a multitude of branches, a stipe and gas-filled vesicles) provided a high surface area for colonisation, which was observed to be highly populated on collection dives. Artificial substrate was selected based on literature documenting high caprellid assemblages32 on aquaculture sea cages and other artificial structures; consequently, the substrate selected was anchor rope, approximately fifteen millimetres in diameter and constructed of polypropylene. Ropes were cut in lengths that allowed a coil formation at the base of the carboy for maximum inhabitable area; each carboy received two pieces of rope suspended in the water.
Water quality parameters were maintained within narrow ranges; tests for pH, dissolved oxygen, salinity and temperature were conducted with a YSI Instruments Multimeter (Ohio, USA), while ammonia levels were tested using a Red Sea Marine Lab Ammonia/Ammonium colourimetric test kit (Texas, USA). Light was provided on a 12:12 schedule at an intensity of 2000 lux throughout the sixteen-week trial. Temperatures were not controlled throughout the trial period, but rather dictated by ambient temperatures of the laboratory, though throughout the duration of the trial, temperature remained relatively constant at 15°C ±2.5°C.
The caprellids were reared on one species of microalgae, being either the green algae Dunaliella tertiolecta, or one of two species of large celled, benthic diatoms, being Cylindrotheca fusiformis and Phaeodactylum tricornutum, which are apparently harvested for feeding by the caprellids by a scraping action.18,45,46 Samples of 50mL of microalgae were drawn up from each algae container with a pipette and added once every second day to the culture vessels, as per the methodologies of Cook et al.45 and Takeuchi & Hirano.18 Axenic cultures of D. tertiolecta, C. Fusiformis and P. Tricornutum were sourced from the Australian National Algae Culture Collection (CSIRO, Hobart, Tasmania), and were cultured in a closed system, in 5L flasks at 25°C, and scaled up to 20L carboys when densities increased. Natural seawater was autoclaved and enriched with F/2 nutrient and F/2 with silica for use as the culture medium. Combinations of D. tertiolecta, C. Fusiformis and P. Tricornutum were fed to the caprellids for the first 10 weeks of trial 1, then the population growth and survivability of the animals was recorded in each substratum. In trial 2, caprellids were cultured on what was found to be the most suitable substratum in each of the culture vessels; three replicate vessels were then used for each algal species, and one for the control, to which no algae was added. The experimental diets included, D. tertiolecta, C. Fusiformis and P. tricornutum.
In each trial, an equal number of ovigerous females were added; as stated by Takeuchi & Hirano,18 male caprellids may be identified by the presence of abdominal appendages, while females are distinguished by the presence of oostegites at pereonites 3 and 4. Sexual maturation in females can be identified from the full formation of oostegites.18
Weekly observations of each female were made to determine whether offspring had been released from the brood pouch. In the case that a female had reproduced, the number of offspring were counted under a stereomicroscope and recorded. Population observations were conducted each week by removing all individuals and counting them. Any dead caprellids were removed and recorded. Performance on each vessel was monitored by caprellid survivability and reproduction throughout the trial. In the first phase of the trial for a 10 week period for the determination of suitable substrates, 50 caprellids were used in each treatment replica. In the second phase of trial 2 to determine food preference and best performance, 20 caprellids were used in each treatment replica. In this case, young caprellids were used, which were produced in the culture vessels during the previous 10 week period.
Each culture vessel had algal diets provided on an ad libitum basis every two days, with any faecal pellets or obvious detritus being removed daily. The performance of each diet was gauged by making an assessment of caprellid survivorship, with each culture vessel being monitored at roughly the same time each day for mortalities, which were removed from the respective culture vessel and recorded. A one-way ANOVA was then used to test for differences in survivorship for each treatment at the conclusion of each dietary trial. Graphs were subsequently plotted using Microsoft Excel 2010 (Seattle, Washington) to compare the population means for survivability on each substrate, and hence determine the significance of the results. Tukey’s multiple comparison tests were then used in pair-wise comparisons to assess where significant differences occurred, as per the methodology of Guerra-Garcia et al..46
Substrate affinities
Trial 1 ran over a 16 week period, with water quality being maintained throughout the duration of the experiment by conducting small water changes and providing constant aeration to each culture vessel, and involved the use of 450 caprellids. The population numbers that featured in each trial were monitored and counted weekly. In the control group, survivability was found to have decreased at first, and was then increased as a result of the release of offspring by some females during weeks 6-8 of the trials. The absence of substrate in the trial evidently gave rise to a curious behavioural trait, namely the formation of a colony, with individuals grouping together by the linking of pereopods 6 and 7. A rather rapid decline in population between weeks 1 and 5 occurred, and was possibly the result of the segregation of groups as a result of handling for other tests (Figure 3). Feed uptake in the control group was relatively high on average, with half of all populations observed found to be full, as evidenced by a full digestive tract, which is relatively easy to observe in many caprellids.

Figure 3 Weekly population changes according to the different substrates utilised in the experiments.
The natural substrate trials were observed to perform the most poorly of all trials, with survivability being relatively very low across all replicate treatments. A more gradual decrease in population numbers was observed for the Sargassum substrate when compared to the control group (Figure 3). This could be attributed to the eventual breakdown and degradation of the natural Sargassum substrate, and a consequent increase in ammonia/ammonium levels.
The artificial substrate composed of polypropylene rope showed the best performance throughout the trial, being the only substrate to see positive growth in population sizes (Figure 3). The survivability on the artificial substrate that was supplied was found to be considerable, with the number increasing to a maximal population. In all replicates, final populations were the result of both reproduction and low mortality. Between the three substrates, there were also significant differences in the population growth of adult C. Penantis(ANOVA, F = 16.81, p < 0.00001), Tukey-Kramer, p < 0.05).
Feeding preference
In trial 2, four treatments lasted for six weeks. In trial 1, caprellids fed with D. Tertiolecta demonstrated 35% survivorship after 6 weeks of exposure to this diet type (Figure 4). In trial 2 and 3, caprellids fed with C. Fusiformis and P. Tricornutum demonstrated 155% and 218% survivorship, respectively, after 6 weeks of exposure to these diet types (Figure 4). In the control treatments, only 5% of the animals survived during the experimental duration, with variation amongst replicate treatments being very minimal. Between the four trials, there were also significant differences in the population growth of adult C. Penantis(ANOVA, F = 13.43, p < 0.00004), Tukey-Kramer, p < 0.05).
It is evident from the series of experiments described that the culture substrate could well be a limiting factor in the successful culture of C. penantis, and that survival and reproduction could only occur to a very limited extent without the presence of a suitable substrate. The lack of an appropriate substrate was demonstrated to cause catastrophic crashes in caprellid cultures. The interesting behavioural adaptation of forming a tight group (particularly in the absence of an appropriate substrate) appeared to confer an advantage with regards to population survivability and feeding. Examination of gut contents uncovered relatively high feed uptake rates throughout the duration of the experiment, and caprellids were readily observed feeding by scraping the surface of the culture vessel, presumably for settled diatoms.
Though initial performance on the Sargassum substrate was high (likely due to multiple feed sources), as the experiment progressed a marked decrease was observed in both health condition and feed uptake. Additionally, there was no reproduction observed in any of the trials. The gradual breakdown of structural integrity of the Sargassum and subsequent decomposition of the substrate would have led to fragmented populations of caprellids and consequently led to a relatively high mortality rate. The use of rope as an artificial substrate appears to have been relatively successful. Though there were a small number of mortalities, the population increases that were observed demonstrate that this substrate shows promise as a possible culture tool and an important aspect of culture methodology. Feed uptake was observed to be the highest of all trials, and remained consistent throughout the duration of the experiment. Upon feeding, caprellids could be observed scraping the substratum for food as well as filtering the algal cells from the water as they extended into the water column with their characteristic praying-mantis style body posturing.
Analysis of feeding habits allowed an increased understanding of the relationship between population growth and substrate affinities. Five individuals were randomly sampled from each trial, where available, and observed for feed uptake. The results were consistent with population growth and survivability, where artificial substrate performed the best, followed by no substrate and natural substrate.
The experiment conducted explored the function of substrate on survival of cultured caprellids, with a focus on determining a suitable culture substrate in order to feed larval or juvenile fish in marine hatcheries. The evidence from this study suggests that the use of artificial substrates, such as rope, was the most suitable culture medium, which over time would be likely to produce high densities of caprellid amphipods. The comparatively high performance of rope as a substrate could relate to its relatively inert nature, and its ability to maintain structural integrity without breaking down or fouling the water, as the natural substrate was observed to do in this instance. Furthermore, ropes meet the requirements of a substrate that can facilitate easy feeding, as they could simply be removed from the culture system and placed into finfish tanks for feeding. Ropes have high surface area, meaning that they could support relatively high densities of caprellids, while at the same time allowing juvenile fish ample access to various sections of the rope. Woods suggested that caprellids were often found at much high densities on artificial substrates than natural ones in many regions of the world;1 with this in mind, it could be imagined that placing ropes in close proximity to artificial structures in the wild may be the most efficient and least labour-intensive way to capture large volumes of caprellids. This process would have to be managed carefully to ensure that the often very strict biosecurity standards that are commonplace in commercial applications for hatcheries are not breached, which could be far easier said than done.
The high feed uptake of populations cultured on ropes was presumably due to the settlement of algal cells on the substrates, allowing easy feeding by caprellids with minimal energy expenditure. The natural substrate used in this series of experiments proved to be unsuitable for culture; the eventual degradation of the Sargassum resulted in high levels of ammonia production as the experiment progressed, and in turn fouled and contaminated the water. Despite the ability of Sargassum to act as a potential food source, or at least as a substrate for various food items such as diatoms to settle on, no benefits were observed, and there was no reproduction in any of the natural substrate trials. The ability to maintain live cultures of marine plants in indoor systems without a benthic substrate for anchorage is limited at best. Additionally, collection of individuals for analysis was comparatively difficult on the natural substrate, as the branched Sargassum provided many escape routes for the caprellids, making culturing on this type of natural substrate more difficult. Despite the natural colonisation of softly surfaced algae such as Sargassum, the ability for artificial substrates to harbour colonising algae can result in significantly higher colonisation by caprellids47 this is particularly evident around marine sea cage farms where caprellids are significant components of biofouling communities on the cage ropes.1
The control group in the study aimed to determine whether a substrate was absolutely necessary for survival of caprellids, based on findings of Hirayama & Kikuchi12 that caprellids spend their entire life attached to the substratum. Findings in this system of experiments indicated that a substrate is clearly not a necessity for survival, which was clearly evidenced by the degree of reproduction observed in one of the replicates, though in natural systems where caprellids are prey items for many species of fish and invertebrate species, the lack of substrate would significantly increase the chance of predation. This may indicate that a substrate plays a crucial role in the lives of caprellids, allowing them to seek refuge by way of shelter and camouflage. This observation is consistent with the findings of two studies,12,47 which found that the need for camouflage is often more important than the nature of the substrate itself.
The behaviour of caprellids forming tight groups (as observed in the control treatments that lacked a substrate) by linking pereopods, has not been well documented. This behaviour, were it to occur to a significant extent in situations where an appropriate substrate was available, could lead to something of a hurdle when attempting to use them as a live feed, as it could be very difficult to separate caprellid cultures based on whether they were in the juvenile or adult stages so that they could then be offered as live feeds to juvenile fish. This could result in a mixture of juveniles and adults being consumed by fish, which could well lead to differential rates of growth in the fish and a lack of uniformity developing in the fish cultures.
Similarly, the maternal care of some caprellids involves newly hatched nauplii remaining attached to the abdominal area of the mother for a period of time. This behaviour was observed in the experiment, and the offspring produced maintained contact with the mother for periods longer than two weeks (Figure 5). Woods1 suggested the use of a minor irritant, such as dilute vinegar, as a means for forcing caprellids off a substrate, or in this case, each other. This method could be explored in order to reduce the time involved in segregating populations, or juveniles from adults, though it no doubt adds to the burden of labour when dealing with such live feed cultures.
It is worthy of note that the mortality rate in some of our trials could have been elevated as a result of the stress and mechanical damage that may have resulted from continuous sampling for gut content analysis and population counts. In a commercial culture situation, these issues would not be observed on a large portion of the population, possibly increasing both survival and reproduction rates.
In this study, Caprellids were observed to feed on all the diets offered in the trial including, Dunaliella tertiolecta, Cylindrotheca fusiformis, and Phaeodactylum tricornutum. Considerably lower growth was observed when the animals were fed with D. tertiolecta, and the highest growth was observed when the caprellids were fed Phaeodactylum tricornutum. The reasons for the lower growth of the animals fed with D. tertiolecta could be the nature of the microalgae, which is motile. It is evident that caprellids can receive food from the substrates in the form of attached diatoms and entrapped detritus45 by scraping of the substratum,46 as was observed in all treatments of the experiment.
A great variety of feeding mechanisms have been reported in the caprellid literature based on the behavioural observations of a few species48,49 or even indirect evidences based on fatty acid composition.50,51 Guerra-García et al.50 found that detritus was clearly the main component of the diet, and, strikingly, the contribution of diatoms was extremely low, differing from previous studies. Caprellids, however, can complete their whole life cycle under laboratory conditions upon an exclusive diet of diatoms;18,26 this was evident in this particular study, where caprellids fed on Phaeodactylumtricornutum showed progressive growth and reproduction affinities, resulting in an increase in population size.
Nakajima & Takeuchi52 also cultured self-sustaining populations of caprellids on a diet of the cultured diatom P. Tricornutumand Artemia nauplii in an exhibition tank in the Port of Nagoya Public Aquarium over a period of five years. Caprella penantis is considered to be opportunistic and has been found to consume macroalage, small crustaceans, diatoms and dinoflagellates as well as detritus.21 This dietary variation is perhaps due to the ecological distribution of the species and subsequent prey availability at different locations. This presents another area open to expansion, in regards to the particular feed on which the secaprellids can be cultured, as well as the resulting nutritional value.
A primary consideration for the culture of live feeds is the challenges and expense they provide to the aquaculturist. Whilst a cost-benefit analysis was outside the realms of this project, the evidence derived from the survivability of most populations indicates a slightly more difficult culture situation than that of currently used live feeds. It seems that the key to culturing caprellids successfully may require large culture vessels maintained by a central filtration unit. For example, Nakajima & Takeuchi52 utilized a biological filter, circulation systems and a heating/chilling unit, in order to maintain self-sustaining populations in a public aquarium display tank for over five years. The use of a centralized filtration system allows a greater degree of control over water parameters, as well as providing a consistent range of environmental conditions. In large-scale culture vessels, several individual substrate pieces could allow populations to flourish over time, and simple removal and replacement of substrate pieces when attempting to culture caprellids could be successful.
Based on the experiment conducted, it could be imagined that caprellids are more suited to culture at relatively small scales as a specialist live feed item for highly valued marine finfish species, rather than on a very large scale for exclusive use as a single feed item. However, based on their nutritional value and the attractive nature of their behavior and swimming motion to a large assortment of predatory juvenile fish, caprellids could be a very suitable supplemental diet item for many fish species. As stated by Woods,1 caprellids would be most suited to supplementing or replacing other traditional feed types. Woods1 also considered the use of caprellids as a feed for high value ornamental species such a seahorses or pipefish. This would have to take into consideration the value retrieved for these animals and whether the relatively high degree of labour involved in production would be compensated for. An additional hindrance towards the culture of caprellids on a commercial scale is the production of live young. Other live feed organisms (such as Artemia and rotifers) produce cysts, which allow them to be sourced in enormous volumes for virtually instantaneous cultures. Because caprellids lack the cyst stage, any organisms that are produced would have to be transported live, and while they would no doubt be able to tolerate some deteriorating water quality that is a part of being in transit for aquatic animals, it is certain that caprellid aquaculture lacks the convenience of the aforementioned live feed organisms.
Furthermore, the true benefit of the inclusion of caprellids in the diet should be investigated for many fish species, as different species require different nutrient profiles for proper development. Baeza-Rojano et al.19 conducted a feeding trial for cuttlefish (Sepia officinalis) hatchlings, in which three feed types were analyzed for growth rates and survival. The experiment compared the mysid shrimp (Mesopodopsis slabberi) with Caprella equilibria and several species of gammarid amphipods. The results were that caprellids provided the lowest growth rates and survival; this was thought to be the result of the hardness of the carapace of the caprellid, possibly affecting digestibility or palatability. Nevertheless, if caprellids were found to suit the nutritional profile of a particular species more than that of, say, Artemia or rotifers, it would be worthwhile exploring the suitability of their culture. For example, Kwak et al.53 found caprellids to constitute up to 20% of the dietary intake of Hippocampus japonicus, a popular ornamental seahorse species that is highly valued. Culture of this particular species could warrant the use of caprellid amphipods, as captive seahorses have been observed to favour caprellids as a food source.1 The use of caprellidsas an alternative aquaculture live feed organism is certainly worth further exploration; for many fish species, caprellids could be used as a partial substitute or complete replacement to the diet if economically viable. As stated by Woods,1 provision of suitable conditions, optimal temperatures and sufficient food supply could easily result in large populations being produced, which are capable of culture at extremely high densities. Further study in regards to caprellid inclusion in fish diets is required in order to realise the full potential of all caprellid species, though preliminary analysis, combined with increased knowledge of culture methods, could provide high volumes of caprellids to marine hatcheries for the commercial culture of larval fish.
This study found that survivability of Caprella penantis in closed systems is highest when cultured on artificial substrates. Artificial substrates provided higher survival rates, lower mortality, and greater feed performance than the use of a natural substrate, or absence of substrate altogether. Natural substrate should not be considered as a substrate for culture of caprellids, due to the process of degradation and the fouling effect it has on the water. The use of rope as both a habitat for caprellid culture and a feeding substrate for fish is a suitable approach to large-scale culture of Caprella penantis being offered the diatom P. tricornutum, and with further development could provide an additional or supplemental feed source to marine hatcheries of many species all over the world.
None.
None.

©2016 Awal, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.