Journal of
eISSN: 2378-3184


Research Article Volume 2 Issue 5
Biological Sciences, University of Essex, United Kingdom
Correspondence: Prasanna Stephan Wijesinghe, Biological Sciences, University of Essex, 9 Cricklebeck, Heelands, Milton Keynes, MK13 7PY, United Kingdom
Received: June 24, 2015 | Published: September 24, 2015
Citation: Wijesinghe PS (2015) Effect of Nanoparticles on DMS Consumption and DMSP Concentration in Aiptasia pallida. J Aquac Mar Biol 2(5): 00044. DOI: 10.15406/jamb.2015.02.00044
Wide use of nanoparticles (NPs) in day-to-day products increases the likelihood of NPs entering and effecting marine organisms as a result of direct emissions and indirect terrestrial runoff. The effects of NPs on dimethylsulfide (DMS) consumption and concentrations of dimethylsulfoniopropionate (DMSP) within the holobiont of cnidarians remain obscure. This study examines the effects of titanium dioxide (TiO2 NP) and capped silver nanoparticles (cAg NP) at 50mg/L concentrations, on the microscopically observations of physiological changes to the tropical sea anemone, Aiptasia pallida (clone CC7), the concentrations of DMS and DMSP of its intracellular symbiont Symbiodinium sp. (A02 and B01) and its surface DMS consuming bacteria.DMS was quantified during NP exposure, and 24 and 96h post exposure using gas chromatography (GC). DMS production was found to be 2.5 fold higher in A02 compared to B01. However, concentrations of particulate DMSP were only observed to increase in the B01 strain when exposed to TiO2 NP (22.90±1.55nmol h-1mg-1) compared to control (11.41±0.05nmol h-1mg-1). A common pattern was identified for the surface DMS consuming bacteria on A. pallida, regardless of Symbiodinium strain or NP treatment. During NP exposure DMS consumption was lower in all NP treatments (0.10±0.05nmol h-1mg-1) in comparison to the controls (0.19±0.19 nmol h-1mg-1). 24h post exposure, DMS consumption was higher (0.27±0.13 nmol h-1mg-1) compared to the controls (0.20±0.22nmol h-1mg-1), with DMS consumption returning to similar rates as controls in NP treatments post 96h exposure. Microscopical observations showed that cAg NP had the greatest adverse effect on both Symbiodinium strains, with approximately 40% decrease in cell density after 24h exposure, and loss of tentacles in A. Pallida being observed only in this NP treatment. This work illustrates the potentially negative impacts of NPs on DMS consumption and concentrations of DMSP in the holobiont of A. pallida, thus emphasising the need for more studies to be conducted on NP effects in the tropical marine environments.
Keywords: Nanoparticles, A. Pallida, Concentrations, Symbiodinium, DMSPt, DMSPp
NPs, Nanoparticles; DMS, Dimethylsulfide; DMSP, Dimethylsulfoniopropionate; FASW, Filtered Artificial Seawater; cAg NP, Capped Silver Nanoparticles; GC, Gas Chromatography; ROS, Reactive Oxygen Species
Nanoparticles (NPs) are used in day-to-day products which can be released into the marine environments from manufacturing processes and poorly managed breakdown or decay of manufactured items. They enter the environment via water sources such as terrestrial run off mainly from coastal urban environments, therefore exposure to marine life it is inevitable. Our knowledge of NPs affect marine organisms is very limited. Studies have shown that NPs can have an antimicrobial effect, majority of studies have been conducted with human pathogenic bacteria,1-3 whilst this is an attractive property of NPs, many non-target effects on bacteria that play crucial roles in environmental cycling, such as sulphur4,5 could be negatively impacted, particularly in the tropical marine system where sulphur plays many biological roles.6-8 Given the poor current understanding of the toxic effects of NPs on marine life, it is crucial for more research to be conducted in this field so such effects can be better forecasted. This study looks at how titanium dioxide Nanoparticles (TiO2NP) and capped silver Nanoparticles (cAg NP) affects dimethylsulfide (DMS) consumption and production in the holobiont of Aistasia pallida, which is extensively studied for coral research9 and observes physical indicators of NP stress. Results from this study consumption and production in the coral holobiont. This will create a better understanding of the potential risks to the holobiont associated with A. pallida when exposed to nanoparticles in aquatic environments.
This study concentrated on the affects of TiO2NP and cAg NPs on the production of DMS by Symbiodinium and consumption of DMS by surface bacteria on the sea anemone species Aiptasia pallida as a holobiont. This study teases apart individual components of the holobiont and investigates how each interlink to balance between DMS consumption and DMSP concentration when exposed to NPs. The aim of this thesis is to investigate how NPs stress affects DMS consumption and DMSP concentration of the Aiptasia pallid holobiont.
The main hypothesis of this study is that DMS consumption will decrease in NP treatments with A. pallida, since NPs have a detrimental effect on bacteria.10 Exposure to NPs should lead to elevated levels of DMS due to reduced bacterial consumption.5 In parallel, this hypothesis further was hypothesised that because NPs produce Reactive Oxygen Species11 less DMS will be produced by the symbiont to maintain higher levels of intercellular DMSP acting as antioxidants.12 This thesis presents the first experimental data concerning the DMS response of the A. Pallid holobiont towards NP contamination.
Making up filtered artificial seawater (fasw)
35g Reef salt (Ocean+ Pro formula, D-D the Aquarium Solution Ltd. Essex) was dissolved in 1 L of reverse osmosis water using a magnetic stirrer for 10 minutes. Salinity was checked using a refractometer (salinity at 34 - 36 ppt). The artificial seawater (ASW)was pumped into an autoclaved 1 L Duran via a 1 L Nagene filter unit with a 0.2um cellulose nitrate membrane filter (WhatmanTM) using a VRC - model 200 - 7.0 rotary vane vacuum pump.
Maintenance of Aiptasia pallida
Aiptasia pallida used in all experiments were derived from the CC7 anemone clone lineage obtained from the Pringle Lab. Anemones were maintained in filtered artificial seawater at 26°C with a light intensity of 30 µEm-2 s-1a photoperiod of 12:12 hours (light: dark) and were fed once weekly with freshly hatched (24-48 hours) Artemiasalina after 24-48 hours of feeding, artificial seawater was changed over and any slime was scrubbed off as needed.
Maintenance of Symbiodinium culture
Symbiodiniumstrains A02 and B01 were grown in sterile 50ml of ASP-8A medium13 in 75cm3NuclonTM treated flask (with blue filter cap) at 26°C in a light intensity of ~140µEm-2 s-1 on a photoperiod of 12:12 hours (light: dark). Both strains were obtained from the Pringle Lab.
Nanomaterials
Capped silver nanoparticles (cAg NP) were capped with methoxy-polyethylene glycol, with a core diameter of 13nm ± 7nm. Titanium dioxide nanoparticles (TiO2 NP) had a specified diameter of <25nm and obtain as a dry power (Sigma-Aldrich Chemie GmbH, Germany).
Analysis of DMS
The following equipment and configuration were used in this work. A Shimadzu GC-2010 Gas chromatography (GC) (Milton Keynes, UK) fitted with a 23m packed chromatography column (inner diameter 0.53mm; J&W Scientific), on-column flame and injector detector was used for gas sample analyse. The temperature settings were: injector 200°C, column 120°C and detector 250°C. The GC gases were: nitrogen 60 cm3 min-1 (carrier gas) and air 70 cm3 min-1 and hydrogen 50 cm3 min-1 (flame gases). All samples were run through trap and purge cryogenic enrichment to concentrate DMS gas, before being flushed into the column. Purge was off during all experimental runs. Data were collected and analysed using GC solutions software program (version 2.30)
DMS Calibration
The DMS concentration range produced during the A. Pallida exposure to nanoparticles were covered by setting up a series of known concentrations of DMSP in NaOH from stock solutions. These standards duplicated in the same size vials used for the experiment. Vials of 4.92ml total volume were used with the final volume of 2ml, with 150µl NaOH pipetted into 1850µl DMSP calibration stock. Production of DMS was initiated by shaking the vial. The vials equilibrated at 26°C for 6 hours. Each vial was run through the trap and purge cryogenic enrichment to concentrate the gaseous DMS sample before running it through the GC to produce a peak area corresponding to the DMS concentration in vial headspace. The calibration showed a linear detector response with 0 - 100nM DMS in the aqueous phase (Figure 1). The linear regression was used to obtain the DMS concentration from the peak areas produced by the cleavage activity of NaOH from DMSP.
Known concentrations of DMSP were added to 2ml of NaOH (headspace of 3ml). These underwent trap and purge cryogenic enrichment to concentrate the gaseous DMS sample. Samples were run through GC to produce a peak area corresponding to the DMS concentration in vial headspace. Two runs were done per known DMSP concentration (n = 2).
Aquatic DMSP is cleaved by NaOH into DMS until the headspace DMS and aquatic DMS has come to an equilibrium, hence concentration of DMS in headspace should be equivalent to concentration of DMSP added (100nM) in control vials as the system is static. However in a dynamic system where there is consumption of DMS in solution by bacteria all rates measured are under estimations due to the lag of DMS in the air constantly diffusing into solution.
Different dms production between Symbiodinium cultures
A known volume of Symbiodinium was taken from culture and counted under a light microscope (Olympis BH-2) using a haemocytometer (Marienfeld, Germany). Cell counts were calculated to give the same number of cells per vial in each Symbiodinium strain, A02 and B01. This was left to incubate for 24 hours at 26°C. DMS production between the strains was analysed using the trap and purge cryogenic enrichment technique as previous described.
Calculating Aiptasia pallida biomass and DMS consumption
Titanium (IV) dioxide (TiO2NP) and capped silver nanoparticles (cAg NP) both at 50mg/L was used. 99% DMS (Sigma-Aldrich Chemie GmbH, Germany) was diluted to 100nM in 100ml of sterile ASW in volumetric flask and shaken well and left to equilibrate for 5minutes. 20µL pipetted into experimental vials (2ml in total) and immediately capped tightly. Nanoparticles and DMS pipetting were done in a systematic staggered matter (Table 1) with 10 minute intervals per vials (this allows for an equal incubation time between vials when run on the GC). Vials were incubated for 4 hours at 26°C with a light intensity of 30 µEm-2 s-1.
|
T runs |
Time |
Experimental runs |
Run in gas chromatography |
|
|
T0 (start) |
8 |
Control - Filtered ASW |
Yes |
|
|
T0 (start) |
8.1 |
Control - Filtered ASW + DMS (100nM) |
Yes |
|
|
Start of systematic staggered time run |
||||
|
T0 (end) |
9 |
Control - Filtered ASW |
No |
|
|
T0 (end) |
9.1 |
Control - Filtered ASW + DMS (100nM) |
No |
|
|
T0 |
9.2 |
Filtered ASW + DMS (100nM) + A. pallida |
No |
|
|
T0 |
9.3 |
Filtered ASW + DMS (100nM) + A. pallida+ 50mg/L (TiO2 NP or Ag NP) |
No |
|
|
4 hours incubation |
||||
|
T1 (end) |
13 |
Control - Filtered ASW |
Yes |
|
|
T1 (end) |
13.1 |
Control - Filtered ASW + DMS (100nM) |
Yes |
|
|
T1 |
13.2 |
Filtered ASW + DMS (100nM) + A. pallida |
Yes |
|
|
T1 |
13.3 |
Filtered ASW + DMS (100nM) + A. pallida+ 50mg/L (TiO2 NP or Ag NP) |
Yes |
|
Table 1 Shows the systematic 10 minute staggered time runs from 9am
T0 (start) were the only vials that were run under the Gas Chromatography (GC) without an incubation period, this provides the true start values of DMS in vials accounting for potential loss by leakage or bacterial consumption. T0 (end) were measured by the GC 4 hours after incubation, thereby taking into account loss between T0 (start) - T0 (end). DMS consumption was then measure on an hourly basis by T0 (end) - T1. Note: these run were done in triplicates, table 1 only shows the summary of one run.
Total and particulate Dimethylsulfoniopropionate (DMSP) concentrations
Methodology for total dimethylsulfoniopropionate (DMSPt) and particulate dimethylsulfoniopropionate (DMSPp) extraction was adapted from15 for A. Pallida were homogenized individually in 1ml of FASW in a pestle and mortar. Further 3ml of FASW was added to increase volume of homogenized material. Cell count on the total diluted homogenate aliquots were conducted immediately under a light microscope (Olympus BH-2) using a haemocytometer(Marienfeld, Germany), simultaneously measuring cell volume using a eyepiece graticule and calibrated ocular micrometer (it was assumed Symbiodinium were spherical, dividing cells were excluded; n = 30 cells per sampling counted). This was used to calculate the intracellular DMSP volume per cell (DMSPp).
2ml of homogenate was filtered through a Whatman GF/F 25mm glass fibre at <100 mm Hg vacuum,16 and placed in 10M of NaOH for 24 hours at 30°C, to allow of DMSP cleavage to calculate DMSPp. Whilst DMSPt was calculated by pipetting 1.85ml of total homogenate into 0.15ml of 10M NaOH in 4.92ml vials and left for 24hours for headspace equilibration at 30°C, these were all analysed for DMS by injecting 0.30ml volume of headspace through the vial septum every 60 seconds in to the GC using a gas-tight syringe (GC setting were as previous described).
Data analysis
All data is presented as the mean ± standard deviation of the mean. Data were analyed using either an unpaired t-test or one way ANOVA when data was normally distributed, or a Kruskal-Wallis test if data failed normality. Significance was taken at p<0.05 using the statistical package R version 3.0.2. All graphical illustration were created using Sigma Plot 10. Some figures were produced using Microsoft Windows Paint version 6.3.
DMS production between A02 and B01 Symbiodinium strains
Both A02 and B01 Symbiodinium strains were normalized to cell counts and incubated in at 26°C to identify which strain had produced more DMS. It was found that the A02 strain produced significantly greater volume of DMS per cell compared to the B01 strain. An unpaired t-test was conducted identified a very significant difference; p<0.001 (Figure 2).
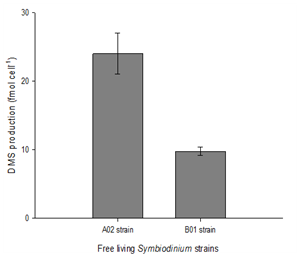
Figure 2 Difference in DMS production in free living Symbiodinium strains (normalized to cell number).
(All data is represented as the mean ± SD (n = 4). Data were analysed using Student's t-test (p<0.05).
Cellular density, diameter and volume between A02 and B01 Symbiodiniumstrains
Cell densities were normalized to dry weight. There was approximately ~40% decrease in cell densities in both strains when exposed to cAg NP treatments compared to control treatments. Cell densities in TiO2NP treatments remained similar to control treatments. A02 control treatment strains had the smallest diameter of 8.0µm3 and a corresponding cell volume of 269µm3 cell-1, and B01 control treatment strain had the largest diameter of 10.2µm3 and a corresponding cell volume of 556µm3 cell-1. Cell diameter and volume decreased in both strains after exposure to either NP treatments (Table 2).
|
Treatment type |
symbiodiniumstrain |
Cell density (cell mg-1) |
Diameter (µm3) |
Cell Volume (µm3cell-1) |
|
Control |
1.0x106± 4.5x105 |
8.0 ± 0.42 |
269 ± 42.7 |
|
|
cAg NP |
A02 |
6.1x105± 1.9x104 |
7.1 ± 0.99 |
193 ± 78.7 |
|
TiO2NP |
1.0x106± 1.9x105 |
7.5 ± 0.21 |
254 ± 20.5 |
|
|
Control |
1.3x106± 2.0x105 |
10.2 ± 0.28 |
556 ± 46.2 |
|
|
cAg NP |
B02 |
7.6x105± 4.6x105 |
9.7 ± 0.49 |
472 ± 72.4 |
|
TiO2NP |
1.5x106± 4.7x104 |
9.2 ± 0.14 |
408 ± 18.8 |
Table 2 Average values for cell density, cell diameter and cell volume in whole organisms of A. pallida in symbiosis with two different strains of Symbiodinium sp. Shown are Symbiodinium per A. pallida means ± SD (n = 2)
Behavioural and microscopical observations
General observations suggested smaller A. pallida were more susceptible to NPs, and in some cases severely comprised, especially to cAg NP treatment. Mucus production and tentacle retraction was immediately observed in both A. pallida strains when exposed to TiO2NP, with the majority of free floating TiO2NP being trapped within the mucus (Figure 3E). After 24h being exposed to TiO2NP some anemones were observed to have partially or full retracted tentacles (Figure 3C,3D) such behaviour was not specific to any one strain. On the other hand, cAg NP initiated mucus production and tentacle retraction notably later in comparison to TiO2NP treatment. A far greater potent effect was observed after 24h of cAgNP exposure, A. pallida displayed extreme stressed symptoms such as: loss of tentacles, bleached tentacle tips, complete bleaching, expulsion of zooxanthellae and bloated body with a thickened epidermis layer (Figure 3G,3H,3I,3F,3D). Darkened areas (Figure 3C,3D,3F) were never observed on controls.
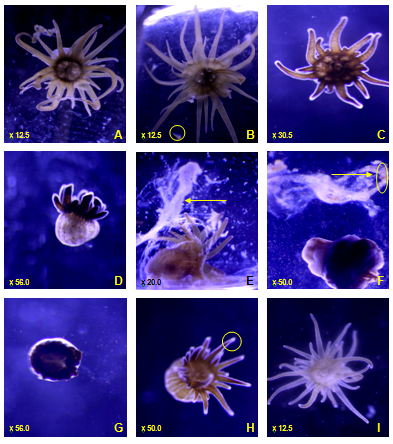
Figure 3 Common physical symptoms of Aiptasia pallida. (A) control A. Pallida (B) control A. pallida feeding on Artemiasalina (free swimming Artemiasalina circled) (C) partially retracted long tentacles (D) all tentacles retracted with thickened epidermis layer and bloated body (E) mucus production (F) mucus production with expulsion of zooxanthellae around A. pallida and trapped in mucus (circled), loss of tentacles, thickened epidermis layer (G) loss of tentacles (H) bleached tentacle tips (I) bleached A. pallida.
Note: magnification on bottom left of images.
DMS consumption after np treatment in surface bacteria
Trend was observed in consumption pattern for DMS consuming bacteria on A. pallida, regardless of Symbiodinium strain or NP treatment. Trend general showed a decrease in DMS consumption following exposure in either NP treatments when compared to the controls. This was followed by a peak in DMS consumption 24h post exposure when compared to the controls, with DMS consumption returning to similar rates as controls in NP treatments post 96h exposure.
A one-way ANOVA was conducted to compare the differences between NP treatments to control treatments on DMS consumption. There were no significant effects on either strain when treated with TiO2NP (Figure 4A,4C). A significant response was found for cAg NP treatments on A02 A. pallida, p<0.05, a post hoc Tukey test identified a significant decrease in DMS consumed 96h post NP (0.02±0.01nmol h-1mg-1 dry weight) compared to control (0.10±0.05nmol h-1mg-1dry weight) p<0.05 (Figure 4B). Similarly, cAg NP on B01 A. Pallida had a very significant effect on DMS consumed p<0.001. Post hoc Tukey test revealed that DMS consumed significantly decreased during exposure (0.15±0.07nmol h-1mg-1dry weight)and 24h post exposure (0.37±0.08nmol h-1mg-1 dry weight) when compared to controls during exposure and 24h post exposure; 0.50±0.09nmol h-1mg-1 dry weight and 0.56±0.05nmolh-1mg-1 dry weight, respectively, p<0.05 (Figure 4D).
Figure 4 shown are means ± SD (n = 3). Data were analysed using one way ANOVA followed by Post hoc Tukey test. Asterisks denote a significant difference from concurrent controls (p<0.05). Note the difference in scales along the y-axes.
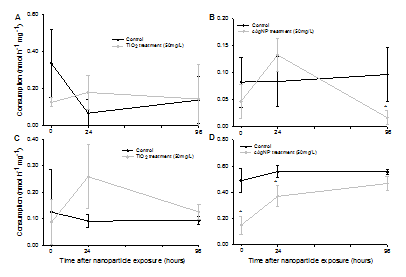
Figure 4 Rate of DMS consumption (normalized to dry weight) during 4h exposure (shown as 0 hours on the x-axes), and after 24 and 96h of exposure. (A) A. Pallida A02 with TiO2 NP, (B) A. Pallida A02 with cAg NP, (C) A. Pallida B01 with TiO2 NP, (D) A. pallidaB01 with cAg NP.
Controls between treatments were found to have different rates of consumption, even between the same strains (Figure 4) after normalization to dry weight. Thus it was hypothesised surface area: volume ratio played a role in such differences. A Pearson's correlation was conducted to test the relationship between oral disc diameter of both strains and DMS consumption at 0 hours. A very significant negative relationship was identified between DMS consumption and oral disc diameter, p<0.001 (Figure 5).
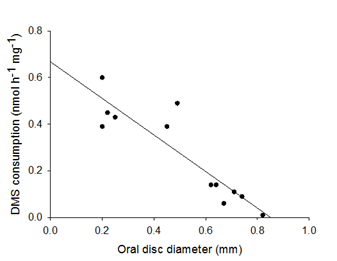
Figure 5 Comparison of oral disc diameter (mm) to DMS consumption (nmol h-1 mg-1) for both control A. pallid Symbiodinium strains.
Data were analysed using Pearson's correlation. Significance was found to p<0.001.
DMSPp and DMSPt concentrations
DMSPt and DMSPp were normalized to dry weight. Concentrations of DMSPp were only observed to increase in the B01 strain when exposed to TiO2 NP compared to control, however DMSPp remained similar to controls when treated with cAg NP. On the other hand A02 treated with either NP treatment decreased in DMSPp when compared to the control (Figure 6A). Furthermore, A02 DMSPp concentrations were greater than B01, this results correspond with results previously described from DMS production between free living strain A02 and B01 strains (Figure 2). Concentrations of DMSPp were generally observed to be lower in either NP treatment for both strains (Figure 6A). A one-way ANOVA was conducted to compare the difference between NP treatment to control treatments on DMS production in DMSPp and DMSPt between the two strain types. No significant response was found in either strain for DMSPp or DMSPt.

Figure 6 DMS produced in DMSPp and DMSPt in A. pallida to 24 hours of exposure to nanoparticle treatment. (A) DMSPp for control, cAgNP and TiO2NP on B01 and A02 A. pallida, (B) DMSPp for control, cAgNP and TiO2NP on B01 and A02 A. pallida.
All data is represented as the mean ± SD (n = 2 for DMSPp, n = 3 for DMSPt). Data were analyzed using one way ANOVA, no significant differences were found to p<0.05. Note: the scale difference between graphs.
Behavioural and microscopical observations
A study on copper oxide NPs16 found these NPs accumulated within the tissue of the host, however, there is no information on the observed physical symptoms of NPs on the host. Results of this study were very similar to the microscopically observed behaviours on A. pallida17 and A. Pulchella18 when exposed to trace metals such as cadmium, cobalt, lead, nickel, copper and zinc, suggesting that the mechanism behind trace metal and NP accumulation are likely to be the similar. Although, the biological reasons behind such behaviours were ambiguously explained. This study provided some suggestions to the biological reasons behind these behaviours. Studies have shown that cnidarian species such as corals produce mucus in turbid waters as a mechanism to remove sedimentation that has settled in the epidermis of the coral, consequently blocking light to the photosynthetic symbiont.19 Instead of sedimentation, NPs that had settled on the epidermis of A. pallida may have triggered the same mucus producing mechanism (Figure 7).
Physiological changes such as a thickened epidermis layer may be to reduce passive entry and accumulation of NPs by osmosis, and bloated body may be to decrease the surface area to volume ratio, thereby decreasing contact area for NP settlement. Tentacle retraction and in other more extreme cases loss of tentacles (which was only observed in cAg NP) may have been due to a greater accumulation of NPs in the symbiont due to its greater tolerates.12 This is reinforced by observations of retracted tentacles to tributyltin (a biocide in anti-fouling paint) in symbiotic anemones compared to no retraction of tentacles in aposymbiotic anemones20 suggested that the host anemones are able to reverse translocate tributylin into the its symbionts, therefore, the loss of tentacles is likely to be a "last resort" mechanism, in which majority of the NPs are reverse translocated from the host to tissue into the symbiont (Figure 7). Furthermore, retracted or loss of tentacles also reduces its surface area. No re-growth of tentacles were observed even after two weeks post NP exposure, thus it is likely that A. pallida was completely autotrophic at this stage (Figure 7). Another mechanism which supports reverse translocation of NPs and was observed is bleaching; the active expulsion of the symbiont by the host.
Additionally, no pedal laceration reproduction was observed during or after NP exposure, which may be due to the detrimental effects of NPs to juvenile marine organisms18,21,22 which may be linked more to the surface area of juvenile organisms compared to adult organisms.
Effects of NPs on consumption of DMS and concentration of DMSP
There was a more pronounced rate of DMS production in the free living Symbiodinium strainA02 compared to B01 in controlled conditions. It was important to establish were there was a difference as this could have played a role in the amount of DMS consumed in surface bacteria. Furthermore, it has been observed that DMS was only produced by the symbiont in A. pallida,4 however if A. pallida utilise DMS is unclear.
A decrease in DMS consumption pattern was observed immediately during exposure to TiO2 NP or cAg NP when compared to control treatments. This suggested that DMS consuming bacteria were either inhibited for the process of DMS consumption or the antimicrobial effects of the NPs severely decreased the surface microbiome population (Figure 7 & 8). The latter argument has been supported by many other studies which have demonstrated that the antimicrobial properties have a negative impact on bacterial populations.10,23 In contrast, 24h after TiO2 NP or cAg NP removal, DMS consumption was observed to peak above across all treatments and strains. Reasons for such a dramatic peak in consumption remains obscure, however, it is unlikely that the bacterial population recovered to normal within 24h of NP removal. This is even more unlikely for DMS consuming bacteria, as such bacteria were observed to have a very long stationary phase when grown on either liquid or solid media (data not shown here). However, it is possible that the antimicrobial properties of the NPs exposed DMS bacterial strains that sensitive to it and possibly selecting for more resistant DMS consuming bacteria, which could have reached the exponential growth phase faster than normal due to the lack of competition and abundance availability of DMS.
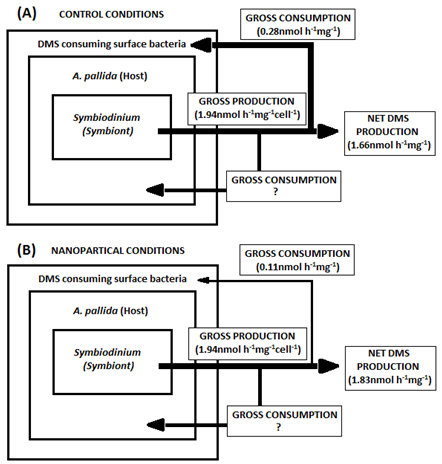
Figure 8 A schematic illustrating the average observed DMS consumption and production in both strains of Symbiodinium in control and both NP treatments.
The other potential explanation comes from observations of bacterial aggregates within the epidermis of the A. pallida (Figure 7). These bacteria were identified as a genus of bacteria called Vibrio,24 although the reasoning behind why such bacteria may exist was not suggested. Interestingly, the Vibrio genera of bacteria were found to consume DMS or DMSP due to its successful isolation when cultured in DMS or DMSP only plates.25 Although, this thesis only suggests possible hypothesis for the peak consumption of DMS, further examination on whether bacterial communities shifted before and after NP treatments may yield interesting results.
Total symbiont per mm2 was derived from.26 Note: due to no significant differences found in DMSPt or DMSPp between NP treatments for either strain in this thesis, the gross production is assumed not to change between control and NP treatment. Furthermore, it is known that DMS only produced by the symbiont in A. pallida,4 however if A. pallida utilise DMS is unclear.
96h post NP treatment saw DMS consume return to similar levels of control treatments. Two possible scenarios can be derived from this, first being the total recovery of all bacterial strains on the surface microbiome of A. pallida, therefore competing for space with DMS consuming bacteria which are likely to be out competed due to their slow stationary phase (data not shown). Second, the Symbiodinium within A. pallida are no longer stressed thus release of DMS levels are of similar rates to control treatments. Resulting in two input sources of DMS; the external experimental input of DMS and the actually DMS production of by the symbiont. The latter was thought to be an artefact of consumption explained by Michaelis-Menten kinetics,27 however, rates of control consumption did not follow the trend described by Michaelis-Menten kinetics.
It was observed that control consumption rates differed between each other between the same strains even after being normalised to dry weight. It was hypothesised that the surface area to volume ratio may play a role in these differences as it does not follow a linear pattern, as expected when DMS consumption was normalised to dry weight. It was found that as oral disc diameter increased DMS consumption decrease, suggesting that the surface area to volume ratio is likely to be a better measurement of DMS consumption compared to dry weight normalisation with sea anemone experimentation. Furthermore, oral disc diameter can be used as a parameter for relative maturity of A. pallida, following the logic that the larger the size of the anemone is the older it is likely to be. Following this suggestion and the fact that more juvenile anemone would have a relatively greater surface area compared to adult anemones. A greater surface area allows for a greater exposure of NPs to juveniles. As previous mentioned no pedal lacteration reproduction was observed, therefore this thesis supports that the detrimental magnitude of NPs may change according to the life stages of A. pallida, other studies have found similar results of other juvenile marine organisms.18,21,22
Such results would suggest that general the type of nanoparticle (capped or uncapped, or type of metal) does not matter, as both had similar negative effects on the surface microbiome (DMS consuming bacteria). Whilst the host (A. pallida), was observed to lose tentacles only when exposed to cAg NPs, the general pattern of behaviour between both NPs used overlapped greatly. However, the symbiont's response between NP treatments were notable different. The general cell volume between strains in each NP treatment was similar to the control treatment. However, cell density in both strains decreased by approximately 40% in cAg NP when compared to control treatments, TiO2 NP treatments did not change in comparison to controls. On the other hand a decrease in cell diameter was only observed in TiO2 NP treatments when compared to controls. A possible reason for the symbiont's varied response to specific NPs may be due to the host acting as a buffer against complete exposure to NPs, which further explains the general uniform effects to both host and DMS consuming bacteria.
It is also possible that the decreased cell density in cAg NP maybe be due to the loss of tentacles. However, it is important to note that the NPs used in this thesis were engineered differently. TiO2NP was not capped, therefore would either settle with time or be trapped within mucus produced by A. pallida. Ag NP were capped, preventing aggregation or effective removal by mucus. Therefore, it was more likely to interact with the host tissue and causing more damage; loss of tentacles, thus, cAg NP acts indirectly in the observed decrease of cell density (Figure 7).
There were no observed differences between control and NP treatments for DMSPp or DMSPt between either Symbiodinium strain. However, DMSPp showed the A02 strain was still the higher DMS producing strain, as DMSPp was higher in A02 compared to B01. Therefore, it can be assumed that DMS production by the symbiont remains the same regardless of NP treatment (Figure 8). Furthermore, other studies have suggested that NPs can produce reactive oxygen species (ROS) within algae and phytoplankton, in relation to this it has been observed that other ROS producing conditions such as UV light, CO2 and Fe limitation were observed to increase cellular DMSP and/ or its lysis to DMS in marine algal cultures28 (Figure 7). Interestingly, DMSPt and DMSPp results from this study conflict with results from.28 This is likely due to the duration of NP exposure, all marine algal cultures were exposed for 9-14 days in ROS producing conditions28 whilst this study only exposed A. Pallida for 24h, thus it is likely that NP exposure was not long enough for ROS species to be produced hence the insignificant differences between and NP treatments. Further studies need to be done to investigate if algae species in a symbiotic relationship respond in a similar way to algae species on their own.
In conclusion, this study provides evidence which supports the hypothesis that NP exposure to A. Pallida will have a detrimental effect on bacteria. It was hypothesized that NP exposure would increase DMSP due to ROS, however no increase of intercellular DMSP was observed after NP treatments. This is most likely due to the short term exposure of NPs (24h), which possibly could have not been long enough for ROS production to occur.
The focus of this study was to assess the affect DMS consumption and DMSP concentrations in the holobiont of A. pallida after being exposed to TiO2NP and cAg NP. However, it is important to gain an understanding of the effects of DMS consumption and production to environmentally-relevant concentrations of a range of NPs to the holobiont of A. Pallid and coral species. In addition, more studies need to be conducted to assess the long-term effects of NPs exposed at environmentally-relevant concentrations and investigate its effects over multiple generations.
I would like to thank Dr. Michael Steinke, Kyle Zawada and William Passfield all the advice they provide throughout this study. Alessandro Moret and Jasmine Sanders for their bacterial cultures. All the members of staff in the labs and individuals that aided me in this study. The University of Essex for all the facilities and resources and the Pringle Lab for providing Symbiodinium strains (A02 and B01) and A. pallida.
None.

©2015 Wijesinghe. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.