Journal of
eISSN: 2378-3184


Research Article Volume 2 Issue 4
1Institute for East China Sea Research, Graduate School of Fisheries and Environmental Sciences, Nagasaki University, Japan
1Department of Aquaculture, Universiti Putra Malaysia, Malaysia
2Eastern Marine Biology Research Center of Fisheries Research Institute, Taiwan
3Graduate School of Fisheries Science and Environmental Studies, Nagasaki University, Japan
4Gunma Prefectural Fisheries Experimental Station, Gunma Prefectural Fisheries Experimental Station, Japan
5Department of Environmental Biology and Fisheries Science, National Taiwan Ocean University, Taiwan
6Coastal and Offshore Resources Research Center of Fisheries Research Institute, Taiwan
Correspondence: Seishiro Furukawa, Institute for East China Sea Research, Graduate School of Fisheries and Environmental Sciences, Nagasaki University, Taira-machi, Nagasaki 851-2213, Japan, Tel 81-95-850-7311
Received: December 26, 2014 | Published: August 18, 2015
Citation: Furukawa S, Chiang W, Watanabe S, Hung H, Lin H, et al. (2015) The First Record of Peritoneal Cavity Temperature in Free-Swimming Dolphinfish Coryphaena Hippurus by Using Archival Tags, on the East Coast of Taiwan. J Aquac Mar Biol 2(4): 00032. DOI: 10.15406/jamb.2015.02.00032
The ambient water temperature and peritoneal cavity temperature of two dolphin fish Coryphaena hippurus, in relation to their vertical movements were examined using archival tags, on the east coast of Taiwan. The peritoneal cavity temperature of the dolphinfish nearly corresponds to the ambient water temperature on a half-day scale, suggesting that they are typical ectothermic fish. This is quite different from Thunnus species, such as big eye, T.obesus and bluefin tuna, T. orientalis, as reported elsewhere. It is known that dolphinfish are predominantly confined to the surface mixed layer, in contrast to the immature bluefintuna that repeatedly dive across the thermocline for short periods; this is when the horizontal distributions of these species overlap. By using a simple heat budget model, we compared the thermo conservation abilities of dolphinfish and immature bluefin tuna, which revealed that the peritoneal cavity temperature of dolphinfish showed more rapid decrease when they dived beyond the thermocline that of the immature bluefin tuna that could maintain a relatively constant body temperature. This is one possible reason why these two species display such markedly different patterns of vertical movement, which is influenced by the thermocline.
Keywords: Dolphinfish; Archival tag; Peritoneal cavity temperature; Heat budget model
Fish behavior is often driven by water temperature and physiological thermal tolerance. The strong correlation between water temperature and habitat selection among pelagic predatory fish has been described on a global scale [1]. The common dolphinfish Coryphaena hippurus is a highly migratory oceanic pelagic fish found worldwide in tropical, subtropical, and temperate waters [2]. It is targeted by both recreational and commercial fisheries throughout its range. Recent biologging studies in the East China Sea revealed that dolphinfish and Pacific bluefin tuna Thunnus orientalis display markedly different patterns of vertical habitat use that are influenced by the thermocline when the horizontal distributions of these species overlap [3]. The vertical movements of dolphinfish were predominantly confined to the surface mixed layer above the thermocline in the East China Sea [4]. On the other hand, immature Pacific bluefin tuna in the East China Sea frequently dive below the thermocline in the daytime, although they remain predominately in the surface mixed layer when the thermocline develops in the summer, a behavior that is probably related to feeding [5,6]. According to some previous archival tag studies of bluefin tuna, the Atlantic bluefin Thunnus can dive to depths of more than 1,000 m while maintaining warm body temperature [7]. Immature Pacific bluefin tuna can also maintain their body temperature when diving beyond the thermocline, although the duration of the dive is limited to a short period [8,9]. From the pattern of vertical movement of dolphinfish in relation with a thermocline [4], we hypothesize that, unlike bluefin tuna, dolphinfish cannot maintain body temperature if they dive beyond the thermocline. The main objective of this study is to test this hypothesis in free-swimming dolphinfish by analyzing the records of the ambient water and peritoneal cavity temperatures from archival tags. Finally, we discuss the reason why dolphinfish do not dive beyond the thermocline [4] with respect to characteristics of thermo conservation exhibited in bluefin tuna [8].
Archival tag
The main body of the archival tag (LAT 1410, Lotek Wireless Inc. LAT1410, New market, Ontario, Canada) used in the present study was 35 mm in length and 11 mm in diameter, with weight (in air) of 5 g. A thin, flexible stalk, 215-mm long, was attached to the main body. The sensors for light and external temperature measurements were embedded atthe end of the stalk, whereas those for pressure and internal temperature measurements were installed in the main body of the tag. The external and internal temperatures and swimming depth were measured at intervals of 120 s (720 data day–1) for approximately 55 days.
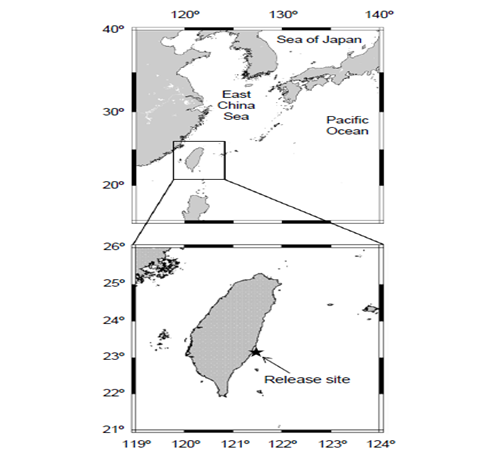
To ensure data retrieval and that our archival tags were easily recovered by fisheries, we chose a study area with high archival tag recovery rates by fisheries after release of tagged fish. We decided to conduct the archival tagging study on the east coast of Taiwan, as it is one of the major sites for migratory fishes, with annual catches of more than 10000 metric tons, accounting for 82% of the total production from entire Taiwan area [10]. Dolphinfish C. hippurus were captured by set net fishing (Figure 1), and 12 with fork lengths (FLs) varying from50 to 113 cm were used for tagging. The archival tags were surgically inserted into the abdomen at 4–5 cm above the anus. The setagged dolphinfish were released immediately at the capture site (Figure 1). According to a breeding experiment for 13 day after tagging, dolphinfish resume feeding within 1–2 days. In addition, data records of the peritoneal cavity (internal) temperature did not indicate the occurrence of negative responses following tag attachment. Of the 12 released dolphinfish, two were recovered on May 18 and 25, 2011, with the data recorded for 4 and 10 days.
Heat budget model
To examine the response of the peritoneal cavity temperature to variations in the ambient temperature, we used the heat budget model [11-13]. The equation for the peritoneal cavity temperature (°C) can be written as
where is the ambient temperature (°C), t is the time (s),
is the whole-body heat-transfer coefficient (s-1), and (
) ̇is the internal heat production (°C.s-1). Time change of
is proportional to the difference between
and
. Since inspection of data from the previous study indicated that large fluctuations in
might occur [14,15], two different possibilities for the value of
were examined:
(2.1)
(2.2)
Where is a threshold value for the difference between
and
. Model (2.1) assumes that thermoregulation is engaged in water cooler than
, and disengaged in water
warmer than
. That is,
when the fish is in heat-retention mode, and
when the fish is in heat-absorption mode [14]. Model (2.2) assumes that the heat-exchange system is always engaged, but the fish is unable to alter
. The parameters for each model were estimated using a numerical procedure that minimizes the squared differences between observed and predicted peritoneal cavity temperatures. The predicted peritoneal cavity temperature
at each time can be written by differentiating Eq. (1) as follows:
where and
are ambient and predicted peritoneal cavity temperatures at each time
, respectively, and
is the time difference (120 s). The gosolnp function in the Rsolnp package of R 3.02 (The R Project for Statistical Computing: http://www.r-project.org/) was used to minimize the residual sum of squares between
and
. To select the best-fit model, we used the Akaike information criterion (AIC), under the assumption of normal distribution for peritoneal cavity temperature at time
. The model that yielded the minimum BIC was selected as the best model.
Vertical movement patterns
The time-series data obtained for dolphin179 (May 15–18, 2011, 54 cm FL at release time) and dolphin 596 (May 16–25, 2011, 73 cm FL at release time) are shown in Figures 2A & 2B respectively. Immediately after the tag-and-release procedure, dolphin179 dived to a depth of approximately 40 m and remained at a depth of between 20 and 45 m. It did not appear at the surface during the first 1.4 h after its release. On the other hand, dolphin 596 also dived to a depth of approximately 30 m but repeated vertical movements from the surface to a depth of 45 m. It did not remain at the surface in the first 5.0h after the tagging procedure. It is a known fact that dolphinfish spend most of their time at the surface [4,16]. Therefore, when the two fish stayed at the surface, we considered that they had recovered from the impact of tagging, and we did not use the post-release data (initial 5.0 and 1.4 hour for dolphin179 and dolphin 596, respectively) due to the irregular behavior. Dolphin179 and dolphin596 spent 79.8% and 83.3% of their time, respectively, at the surface (0 - 5 m), although they made vertical excursions, reaching a maximum diving depth of 62.7 m (dolphin179) and 54.1m (dolphin596) (Figure 2A & 2B). The mean (± standard deviation; SD) swimming depth for the two fish ranged from 5.9 ± 9.8 m (dolphin179) to 5.2 ± 9.9 m (dolphin596).

Ambient water and peritoneal cavity temperatures
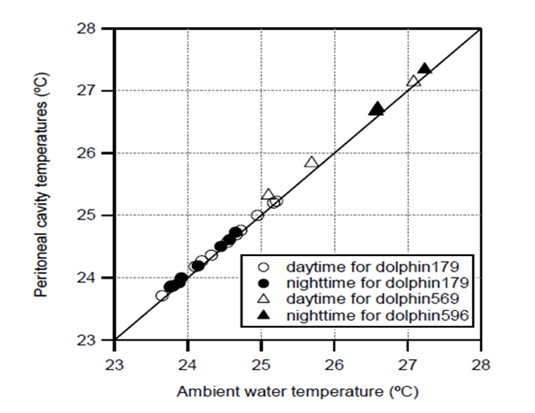
The ambient water temperatures ranged from 19.0 to 26.2°C for dolphin179 and from 20.8 to 27.9°C for dolphin 596. The mean values of ambient temperature were 24.4 ± 0.8°C and 26.6 ± 0.9°C for dolphin179 and dolphin596, respectively. The average peritoneal cavity temperature was similar to the ambient temperature (24.4°C for dolphin 179 and 26.7°C for dolphin596); however, the SD of the peritoneal cavity temperature was smaller than that of the ambient temperature (0.7°C for both dolphinfish). The mean values of the ambient water and peritoneal cavity temperatures were calculated for daytime and nighttime, and their relationship for dolphin 179 and dolphin 596 is shown in Figure 3. The peritoneal cavity temperatures of dolphinfish nearly corresponded to their ambient water temperature on this time scale, and there is no difference between the daytime and night time. The temperature difference between ambient and peritoneal cavity temperature ranged from -2.4 to 5.2°C for dolphin 179 and from -2.9 to 6.0°C for dolphin 596, and the mean values were 0.0 ± 0.4 °C and 0.1 ± 0.6°C for dolphin 179 and dolphin 596 respectively.
Estimation of heat budget model parameters
The heat budget model parameters of bluefin tuna in the East China Sea were estimated when the fish dived from the surface or returned to the surface from the cold water beyond the thermocline [8]. In this study, we could not determine whether a strong thermocline developed, because the vertical profile of the water temperature from the surface to the bottom was not measured at the fishing ground and dolphinfish seldom dive beyond the thermocline [4]. However, the tagged fish encountered a decrease in the ambient water temperature although the vertical movement range appeared primarily limited by a change of 3.7–4.8°C in water temperature relative to the sea surface temperature. When fish encountered low water temperature, decreased rapidly, whereas
decreased relatively slowly on this short time scale (Figure 2C). We estimated the heat budget model parameters using the numerical procedure described by Eq. (2.1) and Eq. (2.2). Eq. (2.1) yielded the minimum AIC. The values of
and
produced by Eq. (2.2) for dolphin179 were
=3.10 × 10-3s-1,
=3.38 × 10-3 s-1,
=1.45 × 10-4°C.s-1 and
=3.27 × 10-2°C. Similar values were obtained for dolphin 596 (
= 2.00 × 10-3s-1,
= 2.23 × 10-3s-1,
̇ = 1.95 × 10-4°C.s-1,
=4.32 × 10-2 °C). It should be noted that there is little difference between
and
values, although different AIC value were observed between Eq. (2,1) and Eq. (2.2). On calculating
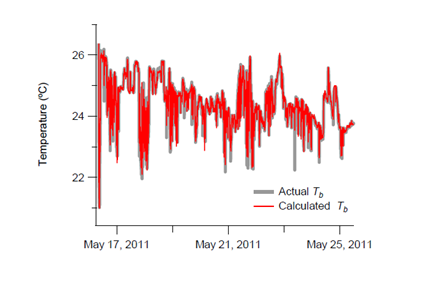
in dolphin179 (grey line) it was found to be closely correlated to the observed peritoneal cavity temperature (red line), as shown in Figure 4. Thus, the peritoneal cavity temperature in dolphinfish can be predicted from the ambient temperature, by using the heat budget model.
To our knowledge, the present study on dolphinfish is the first to record the peritoneal cavity temperature in open sea by using electronic tagging technology. The peritoneal cavity temperature of dolphinfish closely corresponded to the ambient water temperature, suggesting that the peritoneal cavity temperature is greatly affected by ambient water temperature. The heat budget model also revealed this phenomenon in Thunnus species, such as big eye and bluefin tuna. According to Holland et al. [12], big eye tuna undergo physiological and behavioral thermoregulation by changing the heat-transfer coefficient by two orders of magnitude ( = 5.22 × 10-4s for cooling;
= 4.01 × 10-2s for warming). As big eye tuna ascend from cold to warm waters, heat is absorbed rapidly from the warmer water, and when they return to the cold water, the heat is retained in the body. However, in dolphinfish, almost no difference in
values were noted for cooling and in
for warming, suggesting that this does not undergo physiological thermoregulation as does the big eye tuna. In addition, Kitagawa et al. [8] calculated that k in blue f in tuna was similar in both cooling and warming, that this value was smaller than both
and
in dolphinfish by about one order of magnitude, and also that heat production (
) of dolphinfish is smaller than that of immature bluefin tuna. This indicates that dolphinfish rely more on acquiring heat from the ambient water temperature, whereas bluefin tunarely more on internal heat production to increase body temperature like an endotherms [8].
These results suggest that dolphinfish are typical ectothermal fish. Furukawa et al. [3] also demonstrated that the dolphinfish and immature bluefin tuna display markedly different patterns of vertical movement, which are influenced by the thermocline when the horizontal distributions of these species overlap in the East China Sea. Here, we compared the thermo conservation ability of dolphinfish with that of immature bluefin tuna, in terms of peritoneal cavity temperature change, on diving beyond the thermocline. Integration of equation (1) results in:
Where Te is the body temperature at the steady state. Equation (4) indicates that body temperature changes exponentially when ,
, and
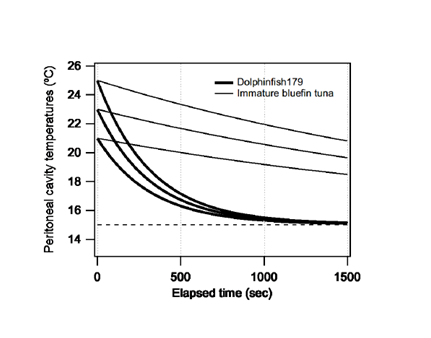
are constant.We simulated peritoneal cavity temperature change when fish dive beyond the thermocline from the surface water by using Eq. (4). We assumed initial peritoneal cavity temperatures of 21, 23, and 25°C respectively and a constant ambient water temperature (15°C). The time series for peritoneal cavity temperature calculated using Eq. (4) are shown in Figure 5. For dolphinfish, we further specified
as 3.20 × 10-3s-1and
as 1.74 × 10-4 °C.s-1, which are the values used in Figure 5 and referred to by Kitagawa et al. [8] for bluefin tuna. It is noticeable that the immature bluefin tuna was able to maintain body temperature for a short period, but the body temperature of dolphinfish rapidly decreased when they dived beyond the thermocline. This difference in the cooling process is attributed to the differences in
values between bluefin tuna and dolphinfish; the latter has a smaller
value by about one order of magnitude, resulting in much lesser insulation. It is expected that if dolphinfish dive from warm to cold ambient temperatures across the thermocline, over a short period, their peritoneal cavity temperature will be as cool as the ambient temperature because of the heat-transfer coefficient of the fish. This could be why dolphinfish do not dive beyond thermoclines.
The adaptive significance of thermo conservation ability is discussed further. Some hypotheses have been proposed about the ecological or physiological importance of fish being endothermic: the ability to maintain temperature may enable rapid digestion, rapid growth, and rapid recovery from exhaustive exercise [17-25]. On the other hand, an alternative hypothesis exists stating that heterothermy is a characteristic for high-performance swimming [25]. Furthermore, insulation of bluefin tuna is effective in maintaining body temperature; however, the fish lack thermoregulation ability, and once the body temperature of the bluefin decreases, it will take a long time to recover, as indicated by numerical simulation [8]. An overheating problem was postulated by Neill et al. [26] for the skip jack tuna Katsuwonus pelamis. The activities of this species are probably limited in warm waters as large thermal inertia and high metabolic rate in the body might cause overheating (hyperthermia), as tuna body mass increases with growth.
Dolphinfish are distributed worldwide, particularly in tropical and subtropical waters [2]. Although the dolphinfish feed on diverse prey, these are generally surface swimmers [27-29]. According to Kojima [27], adult dolphinfish feed mainly on pelagic fishes such as Engraulis japonicus, Sardinops melanostictus, and Exocoetidae species in the southwestern Sea of Japan, adjacent to the northern East China Sea. E. japonicus (anchovies), in particular, are predominant in the dolphinfish diet in the southwestern Sea of Japan during summer [27]. These are pelagic fish that aggregate on the surface waters of the East China Sea, during summer [30]. These anchovies may also be restricted to the surface when the thermocline becomes steep. Considering these phenomena, the lack of thermo conservation ability in dolphinfish is probably an adaptive strategy to warmer surface water in contrast to endothermic fish, such as bluefin tuna, that forage across the thermocline.
We thank Mr. W.C.Wu, owner of the set nets at eastern Taiwan, and his crew for their help in tagging and releasing dolphin fish. We also thank Mr. S.C. Chen, Ms. H.H. Hsu, Mr. C.F. Chen, Mr. S. Saeki, Mr. Inoue, Ms. H.L. Lin, Ms. T. Chang, Mr. W.Y. Chen and Ms. M. Chen for their assistance. This study was supported by the JSPS KAKENHI Grant Number (19380114), the Fisheries Research Institute, Council of Agriculture, Taiwan, the Sustainable Aquatic Food and Environment in the East China Sea- PROJECT (SAFE-PROJECT), and the JSPS Bilateral Joint Research Projects with Taiwan (Open Partnership) to R.K.

©2015 Furukawa, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.