Journal of
eISSN: 2378-3184


Research Article Volume 1 Issue 1
1Marine Innovation Southern Australia, Australia
2Flinders University, Australia
3University of Adelaide, Australia
4Fisheries and Aquaculture Division, Primary Industries and Regions South Australia, Australia
Correspondence: David AJ Stone, South Australian Research and Development Institute Aquatic Sciences Centre, Marine Innovation Southern Australia, West Beach, SA 5022 Australia
Received: October 27, 2014 | Published: November 20, 2014
Citation: Stone DAJ, Roberts SD, Currie KL (2014) Hyper-Saline Conditions Affect Growth, Osmoregulation and Survival of Fingerling and Juvenile Yellowtail Kingfish, Seriola Lalandi. J Aquac Mar Biol 1(1): 00005. DOI: 10.15406/jamb.2014.01.00005
Commercial production of yellowtail kingfish (YTK), Seriola lalandi, in South Australia was carried out at Port Lincoln, Arno Bay and Fitzgerald Bay in Spencer Gulf. There are marked differences in the seawater salinity between these locations (35 to 42g L-1). Slower growth rates of YTK have been reported at sea-cage grow-out sites at Fitzgerald Bay than at Arno Bay, and the cause was suspected to be the hyper-saline conditions. The aim of this study, was to determine whether increased salinity (Experiment 1: Fingerlings, 8.6 g, 37, 41 and 45 g L-1 at 24 °C; Experiment 2: Juveniles, 373.5 g, 39, 41 and 45 g L-1 at 20 °C) affected the growth, survival and osmoregulatory capacity of fingerling and juvenile YTK grown at water temperatures corresponding to those they are exposed to during each stage of production. Hyper-saline conditions of ≥41 g L-1at either 24 °C or 20 °C were suboptimal for fingerling and juvenile YTK, respectively. Increasing salinity above 37 g L-1 led to reductions in growth, feed intake and feed conversion ratio, and ultimately survival (37>41=45 g L-1; P<0.05) of fingerlings. Increasing salinity levels also affected growth performance, feed utilisation and survival of juvenile YTK, but to a lesser extent. Osmoregulatory parameters combined with survival data, suggest that juvenile YTK were more tolerant to hyper-salinity than the fingerlings. However, results suggest that salinity levels of 45 g L-1 are close to the physiological tolerance limitation for YTK of these size classes at the temperatures tested in this study. Additional stressors including husbandry practices (e.g. high stocking densities), disease and other environmental parameters (e.g. sub-optimal water temperatures) would contribute to reduced salinity tolerances. It is recommended that YTK fingerlings and juveniles should be stocked at sites of ambient (more oceanic) salinities and within preferred temperature limits (<24 °C).
Keywords: Yellowtail kingfish, Seriola laland, Salinity, Osmoregulation, Growth, Temperature, Survival
YTK, Yellowtail kingfish; CST, Clean Seas Tuna; SARDI, South Australian Research and Development Institute; ASC, Aquatic Science Centre; ANOVA, Analysis of Variance; SNK, Student Newman-Kuels
Commercial production of yellowtail kingfish (YTK), Seriola lalandi, was carried out at three sites in Spencer Gulf, South Australia by Clean Seas Tuna (CST). Water temperatures at these sites can fluctuate from 10 °C in winter to 24 °C in summer.1 The sites are located in the vicinity of Port Lincoln, Arno Bay and Fitzgerald Bay, which stretch from the mouth to the head of the Gulf. As such, there are marked differences in the seawater salinity between these sites (Figure 1). Due to minimal freshwater input (Port Lincoln, 400 mm y-1; Arno Bay, 300 mm y-1; Fitzgerald Bay 200 mm y-1 annual rainfall2) and high evaporation rates (Port Lincoln, 1400 mm y-1; Arno Bay, 1600 mm y-1; Fitzgerald Bay 2000 mm y-1 annual evaporative loss,3), Spencer Gulf is an inverse estuary, with salinity increasing towards the head of the gulf.4 For example the salinity levels at Fitzgerald Bay, the most northerly CST site, may range from 39 to 42 g L-1as compared to 36 to 37 g L-1 at Arno Bay and 35 to 36 g L-1 (ambient oceanic salinity) at Port Lincoln located in mid and southern Gulf waters, respectively. Slower growth rates of YTK in sea-cages have been observed at Fitzgerald Bay compared to Arno Bay (Personal communication, Mike Thompson, Research and Development Manager, CST). The cause is suspected to be the hyper-saline conditions as the life-history of this species is pelagic. Juvenile YTK are found, often associated with flotsam and floating seaweed in oceanic surface waters, in conditions that are of ambient salinity (35 g L-1).5
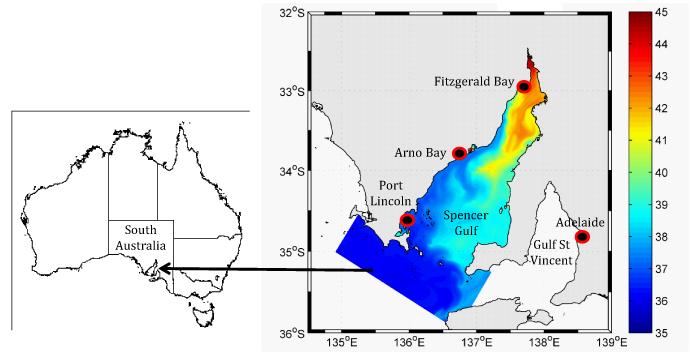
Figure 1 Summer sea surface salinity levels (mg L-1) of sites of yellowtail kingfish sea-cage production in Spencer Gulf, South Australia, Australia (CSIRO “CARS 2009” dataset supplied by Dr John Luick , SARDI).
Numerous studies have shown that 20 to 50% of the total energy budget of fish is dedicated to osmoregulation6 However, more recent studies have indicated that the osmotic energy cost is only 10%.7,8 In addition, Boeuf and Payan6 reported that the stimulation of food intake and food conversion efficiencies are both dependent on the environmental salinity, and that temperature and salinity have complex interactions. They also reported that hormones, such as growth hormone and insulin-like growth factor-1, are active in both osmoregulatory and growth regularity pathways, and as a result, it is likely that multiple causality is responsible for the interactive effects of salinity on physiology and behaviour. It is unknown whether or not increased salinity slows the growth of YTK and if so to what extent.
The effects of salinity on the physiology of marine fish has been extensively studied, with the majority of literature concentrating on euryhaline species and large salinity differences such as freshwater to seawater transfer and vice versa.9-12 Changes in salinity primarily affect osmoregulation and ion regulation in fish, which can be adjusted as a compensatory mechanism to maintain ionic homeostasis if the change is within physiological tolerance limits. Furthermore, the physiological effects of changes in salinity can be augmented as a result of disease and temperature changes.12-14 Measurable physiological parameters associated with osmoregulation include: the mucous layer, gill morphology (i.e. mucous and chloride cell abundance), gill enzyme activity (i.e. Na+/K+ ATPase and succinic dehydrogenase, SDH) and blood plasma electrolyte concentration11-13,15
The aim of this study was to determine whether increased salinity levels affects growth and the osmoregulatory capacity of fingerling and juvenile YTK grown at 24 and 20 °C, respectively; the temperatures where YTK growth, feed utilisation and survival were expected to be approaching optimal.16
Experimental Animals
Eighteen hundred fingerling YTK (mean initial fish weight 6 g), provided by CST from their Arno Bay hatchery (water temperature 17-18 °C; salinity 36-37 g L-1), were transported by truck to the South Australian Research and Development Institute (SARDI) Aquatic Science Centre (ASC), West Beach, South Australia in mid-November 2008. Upon arrival at SARDI the YTK were transferred to, and held in two 5000 L tanks supplied with flow-through seawater (water temperature 18-19 °C; ambient salinity 37 to 39 g L-1; dissolved oxygen levels >7 mg L-1) and fed a Ridley Marine Start commercial diet (50% protein, 25% lipid; Ridley Aquafeeds, Deception Bay, Queensland, Australia) to apparent satiation three times per day. The YTK were held at ambient water temperatures in this system for three weeks prior to the stocking of Experiment 1 and a further five months before the stocking of Experiment 2.
Experimental Design
The two experiments were designed to investigate the effects of a range of nominal seawater salinity levels (Experiment 1: ambient 37, 41 and 45 g L-1; Experiment 2: ambient 39, 41 and 45 g L-1) on the growth performance and osmoregulatory capacity (gill and blood plasma analyses) of fingerling and juvenile YTK. The salinity levels tested are similar to, and above those, which are commonly found between the three CST sea-cage production sites in Spencer Gulf (Port Lincoln; Arno Bay; and Fitzgerald Bay); while the water temperatures are in the range experienced by fingerling (24 °C) and juvenile (20 °C) YTK over the first summer following transfer into the sea-cages at these sites. The ambient salinity levels in the laboratory at SARDI ASC varied between Experiment 1 and Experiment 2 due to fluctuations in incoming Gulf St Vincent (Figure 1) seawater during summer (8 December 2008 to 4 February 2009) and autumn/winter (7 May 2009 to 8 July 2009). Initially, both experiments were planned to run for 84 days. However, due to high mortalities in salinity treatments (41 and 45g L-1) in both experiments, Experiment 1 was terminated after 59 days and Experiment 2 after 63 days.
Experimental System
The experiments were carried out in the nutrition laboratory at SARDI ASC. The re-circulating systems used for the experiments were housed in a temperature and photoperiod (14 h light: 10 h dark) controlled insulated room and comprised three separate identical units (treatment lines) each consisting of a 780 L sump (Nally Megabin MS7800; Nally), electric pump (Davey Pool Master-250; Davey Water Product Pty. Ltd. Scoresby, Victoria, Australia), sand filter (Davey Crystal Clear FG 28), 780 L moving bed bio-filter containing 0.3 m-3 Kaldnes K1 media (Kaldnes Miljoteknologi AS, Tonsberg, Norway) and four 700 L cylindrical culture tanks. The four 700 L conical tanks per salinity treatment were arranged, randomly throughout the room in a manner that enabled water to be pumped from and returned to the respective sump by gravity flow via a network of PVC pipes. To stop fish escaping from the system the top of each 700 L culture tank was fitted with Sarlon 10 mm square plastic bird netting (Donaghys, Brunswick, Victoria, Australia).
The re-circulating systems were supplied with UV treated, sand-filtered seawater drawn from Gulf St Vincent (Experiment 1, 37 g L-1; Experiment 2, 39 g L-1). To achieve the desired treatment salinity levels within each treatment the incoming treated seawater was used either directly, in the case of the ambient salinity treatment, or adjusted to 41 or 45 g L-1 using synthetic sea salt (Ocean Nature Sea Salt; Aquasonic Pty Ltd, Wauchope, New South Wales, Australia). Water exchange to the system was carried out daily by replacing 15% of the culture water in each treatment line with a new batch (700 L) of salinity and temperature adjusted seawater.
Stocking of Experiments
Prior to the commencement of YTK stocking, the water temperature in the experimental tanks was lowered to match the ambient water temperature in the outside holding tanks (Experiment 1, 19 °C; Experiment 2, 18 °C). YTK were then captured by dip net and anaesthetised using 40 mg L-1 of Aqui-S® (Aqui-S New Zealand Ltd, Lower Hutt, New Zealand) in a plastic container in 80 L of oxygenated ambient temperature seawater. To ensure a narrow weight range for stocking, a sub-sample of 30 YTK for each experiment were weighed (0.1 g), fork length measured (0.1 cm) and a frequency histogram based on fish weight was developed and used to determine the required fish stocking size range. Fish were then individually screened from a larger population and weighed, measured, selected and transferred directly into each experimental 700 L culture tanks. This process was repeated until each of the twelve experimental tanks contained 20 individual YTK (mean weight 8.6 g; mean fork length 8.5 cm) in Experiment 1 and 12 individual fish in Experiment 2 (mean weight 373.5 g; mean length 29.6 cm). Four replicate culture units were previously randomly allocated to each salinity treatment. The water temperature was then slowly increased by 0.5 °C d-1 until the nominal temperatures of 24 or 20 °C were reached for Experiments 1 and 2, respectively. These water temperatures were then maintained throughout the remainder of the experiments.
Feeding and Tank Cleaning
In Experiment 1, YTK were fed the same sinking 3 mm diameter pellet (Ridley Aquafeed Marine Start diet, 50% protein, 25% fat) as used during the holding period. For Experiment 2, a Skretting sinking 4 mm diameter pellet marine fish diet (Nova ME, 45% protein, 20% lipid; Skretting, Rosny Park, Tasmania, Australia) was used. For both experiments YTK were fed to apparent satiation twice daily (8.30 am and 3.30 pm daily). Fish in Experiment 1 were fed six days a week (excluding Sundays). Fish in Experiment 2 were fed six days a week from 9 May 2009 to 7 June 2009 (excluding Sundays) and then seven days a week from 14 June 2009 for the remainder of the trial. Feeding typically took between 5 and 10 minutes for each tank at each feeding event. This feeding method was chosen to allow all fish the opportunity to express their full growth potential. Feed intake was weighed and recorded daily for each tank. The bottom drain pipe of each tank was flushed for 30 s daily to remove sedimentation and cleaning of the tanks to remove faecal waste was carried out once weekly.
Sampling of Fish
The weight and fork lengths of all YTK were measured at stocking and also when sampled at the end of each experiment. Note that in Experiment 2, weight and fork length was sampled at day 56, as the end time point, while blood and gills were sampled at day 63. All sampling for blood and gills was lethal, and entailed fish of “unstressed” appearance being captured with a dip net and killed with a blow to the cranium. For blood plasma and analyses, 12 fish were sampled from holding tanks (day 0, initial “ambient salinity controls”) prior to stocking experimental tanks, while a further 12 fish (3 from each of 4 replicate tanks) were sampled from each treatment at day 1 and at harvest (Experiment 1, day 59; Experiment 2, Day 63).
Gill enzyme activity and chloride cell abundance were also determined from subsets of these fish from the ambient and 45 g L-1 salinity treatments for both experiments. The removal of fish at day 1 for blood and gill tissue sampling resulted in 17 and 9 fish being left in each tank for the duration Experiment 1 and 2, respectively. Initial weight, fork length and condition index data presented in Tables 1 and 2 are based on the corrected data for these fish.
|
Performance indices1 |
Salinity (g L-1) |
Probability |
||
|
|
37 (ambient) |
41 |
45 |
|
|
Initial biomass (g tank-1)2 |
147.9 ± 0.62 |
148.7 ± 0.50 |
148.9 ± 0.88 |
0.588 |
|
Initial individual weight (g fish-1)2 |
8.70 ± 0.04 |
8.75 ± 0.03 |
8.76 ± 0.05 |
0.588 |
|
Initial fork length (cm)3 |
8.5 ± 0.31 |
8.5 ± 0.31 |
8.5 ± 0.31 |
na |
|
Initial condition index3 |
1.38 ± 0.07 |
1.38 ± 0.07 |
1.38 ± 0.07 |
na |
|
Final corrected biomass (g tank-1)4 |
1733 ± 45a |
1327 ± 97b |
1073 ± 36c |
<0.001 |
|
Final corrected biomass gain (g tank-1)4 |
1585 ± 46a |
1178 ± 97b |
925 ± 36c |
<0.001 |
|
Final individual weight (g fish-1) |
110.3 ± 4.7a |
88.9 ± 4.1b |
72.5 ± 2.4c |
<0.001 |
|
Final individual weight gain (g fish-1) |
101.6 ± 4.7a |
80.1 ± 4.2b |
63.7 ± 2.3c |
<0.001 |
|
Final fork length (cm) |
19.9 ± 0.24a |
18.6 ± 0.26b |
17.7 ± 0.22c |
<0.001 |
|
Final condition index |
1.39 ± 0.03 |
1.37 ± 0.03 |
1.30 ± 0.02 |
0.117 |
|
Specific growth rate (% body weight-1 d-1) |
4.09 ± 0.06a |
3.73 ± 0.08b |
3.41 ± 0.08c |
<0.001 |
|
Feed consumed (g tank-1 wet weight) |
2031 ± 59a |
1644 ± 107b |
1534 ± 56b |
0.003 |
|
Apparent FCR |
1.28 ± 0.03a |
1.40 ± 0.03b |
1.66 ± 0.01c |
<0.001 |
Table 1 Growth performance and feed utilisation of fingerling yellowtail kingfish cultures at three increasing salinity levels at 24 °C for 59 days in Experiment 1
1Values (mean ± standard error of the mean, n = 4) in the same row that share the same superscript are not significantly different (ANOVA; P>0.05; SNK) 2Initial data based on 17 fish per tank 3Reported initial fork length data and condition index data based on data obtained from fish (mean standard ±deviation; n = 12) used for initial plasma osmolality and gill tissue structure sampling 4Final corrected biomass gain accounts for the weight of mortalities removed
|
Performance indices1 |
Salinity (g L-1) |
Probability |
||
|
|
39 (ambient) |
41 |
45 |
|
|
Initial biomass (g tank-1)2 |
3411 ± 7.7 |
3387 ± 11.4 |
3408 ± 7.6 |
0.175 |
|
Initial individual weight (g fish-1)2 |
379. ± 0.86 |
376.3 ± 1.26 |
378.7 ± 0.85 |
0.175 |
|
Initial fork length (cm)3 |
29.6 ± 0.14 |
29.6 ± 0.09 |
29.6 ± 019 |
0.980 |
|
Initial condition index3 |
1.39 ± 0.02 |
1.40 ± 0.01 |
1.38 ± 0.03 |
0.776 |
|
Final corrected biomass (g tank-1)4 |
5810 ± 98a |
5365 ± 87b |
4941 ± 57c |
<0.001 |
|
Final corrected biomass gain (g tank-1)4 |
2399 ± 105a |
1798 ± 83b |
1533 ± 51c |
<0.001 |
|
Final individual weight (g fish-1) |
646 ± 10 |
626 ± 32 |
585 ± 20 |
0.202 |
|
Final individual weight gain (g fish-1) |
267 ± 12 |
250 ± 32 |
206 ± 19 |
0.199 |
|
Final fork length (cm) |
356 ± 2.1a |
356 ± 2.8a |
345 ± 3.6b |
0.025 |
|
Final condition index |
1.44 ± 0.03 |
1.42 ± 0.02 |
1.42 ± 0.01 |
0.850 |
|
Specific growth rate (% body weight-1 d-1) |
0.97 ± 0.03 |
0.92 ± 0.09 |
0.79 ± 0.06 |
0.198 |
|
Feed consumed (wet basis g tank-1) |
4309 ± 196a |
3672 ± 208b |
3280 ± 76b |
0.007 |
Table 2 Growth performance and feed utilization of juvenile yellowtail kingfish cultured at three increasing salinity levels at 20 °C for 56 days in Experiment 2
1Values (mean ± standard error of the mean, n = 4) in the same row that share the same superscript are not significantly different (ANOVA; P>0.05; SNK) 2Initial data based on 17 fish per tank 3Reported initial fork length data and condition index data based on data obtained from fish used for 24 h plasma osmolality and gill tissue structure sampling 4Final corrected biomass gain accounts for the weight of mortalities removed
Analytical Measures of Osmoregulation
Osmoregulatory capacity of experimental fish was assessed by analysing: 1) Blood plasma osmolality; 2) Gill enzyme activity associated with ionic regulation; and 3) Gill chloride cells.
Blood samples were collected using 29-gauge needles (1 cm long) into 5 mL heparinised syringes (lithium heparin 100 IU mL–1, Sigma) from the caudal vein of each fish. Blood was then transferred into 1.5 mL Eppendorf tubes and stored on ice. Tubes were then spun in a microcentrifuge for 7 minutes; plasma was separated and stored at -80 °C. Blood plasma osmolality was measured at the University of Tasmania using a VAPRO® vapour pressure osmometer (model 5520, WESCOR®).
Gills were dissected out and the second left gill arch from each individual fish was transferred into 50 mL pots of neutral buffered formalin; after 24-48 h the fixative was replaced with 70% ethanol. Fixed second left gill samples were analysed for gill chloride cell numbers. The remaining gill arches of each fish were transferred into a small zip lock bag, wrapped in alfoil, and immediately snap frozen in liquid nitrogen, then stored at -80 °C for subsequent analysis of gill enzyme activities. Analyses of gill chloride cells and enzyme activity (Na+/K+ ATPase and succinic dehydrogenase, SDH) were conducted at the University of Tasmania following methods outlined in McCormick17 and Powell et al.11 Haematoxylin and eosin (H&E) stained histological slides were viewed under light microscope to determine chloride cell abundance (cells per gill filament). Na+/K+ ATPase activity was determined from processed (homogenised and centrifuged) gill tissue in the presence or absence of 0.5 mM ouabain (Sigma) at 25 °C and absorbance was measured at a wavelength of 340 nm. Succinic dehydrogenase was determined using the spectrophotometric technique at 492 nm wavelength. Calculation of Growth Performance Indices
All fish were individually weighed and measured (fork length) at the commencement and completion of each experiment. Growth performance indices were calculated as follows:
Total tank biomass weight gain = (final weight + ∑mortality weight) – initial weight;
Condition index = (fish weight (g) / fork length3) x 100;
Apparent feed conversion ratio (FCR) = amount of wet feed consumed (g tank-1) / total tank biomass weight gain (g tank-1)
Mean individual fish weight gain = final weight of individual living fish – initial weight of individual fish; and
Specific growth rate (g fish-1 d-1) = 100*(ln(final living individual fish weight)– ln(initial individual weight)) / time (d).
Water Quality and Mortalities
Throughout the study water quality was maintained in each experiment at levels considered appropriate for good growth of YTK. Salinity was determined to 1.0±0.5 g L-1daily using a hand held refractometer (Model UR-2, ISSCO, Concord West, NSW, Australia) and was maintained at the required treatment level (Treatment 1, ambient salinity, Experiment 1, 37 g L-1; Experiment 2, 39 g L-1; Treatment 2, 41 g L-1; and Treatment 3, 45 g L-1) for all tanks throughout both experiments. Dissolved oxygen and water temperature were measured daily using a dissolved oxygen meter (Handygamma, OxyGuard International A/S, Birkerød, Denmark). Dissolved oxygen ranged from 7.0 to 9.8 mg L-1 and from 6.7 to 8.9 mg L-1 for Experiments 1 and 2, respectively. Dissolved oxygen did not differ between treatments on any given day. Water temperature remained constant between tanks and ranged from 20.0°C at stocking to 23.0 to 24.6°C for Experiment 1 and 20.0 to 21 °C for Experiment 2. Culture water pH was measured weekly and ranged from 7.51 to 8.06 in Experiment 1, and from 7.30 to 8.08 in Experiment 2. Ammonia (NH4+/NH3 mg L-1) was recorded weekly for both experiments and levels always remained below 0.25 mg L-1. YTK mortalities were removed from tanks and measured, weighed and recorded daily.
Statistical Analyses
Homogeneity of variances among mean values was assessed using Levene’s test for equality of variance errors. Survival was assessed using Kaplan-Meier analyses with Log-rank and Breslow tests, and Cox proportional-hazards regression analyses. Osmoregulation, growth and feed utilisation data was analysed using one-factor Analysis of Variance (ANOVA). Student Newman-Kuels (SNK) test was used to identify significant differences among multiple treatment means. A significance level of P<0.05 was used for all statistical tests, and tank mean values (n = 4) were considered units of observation for statistical analysis. All statistical analyses were done using SPSS, Version 16.0.1 - 18.0 for Windows (SPSS Inc., Chicago, Illinois, USA). Unless otherwise stated all values are presented as means ± standard error of the mean.
Mortality
In Experiment 1, the survival of fingerlings held at salinity levels of 37 g L-1 was significantly greater than at 41 or 45 g L-1 (Log-rank test, P = 0.003; Breslow tests, P = 0.004; Figure 2). There was no significant difference in the survival of fingerlings held at salinity levels of 41 or 45 g L-1 (Log-rank test, P = 0.366; Breslow tests, P = 0.381). Fingerlings held at salinity levels of 41 or 45 g L-1 were 2.4 (Cox regression analysis, P = 0.038) or 3.2 (Cox regression analysis, P = 0.005) times more likely to die, respectively, when compared to those held at 37 g L-1. At the completion of Experiment 1, the mortality of the fish held at the ambient salinity was much lower than the other treatments (11.8%), and was only slightly above the normal range (5-10%) expected when conducting growth trials with YTK in re-circulating systems.16,18-19 Mortalities appeared to follow a similar trend amongst treatments towards the later part of the study. Fish exhibited signs of a gaping mouth, flared gills, circular swimming near the surface of the tank, reduced feed intake, and reduction in body condition index, as displayed in Table 1, and ultimately death. At week eight fish were treated with oxytetracycline under veterinary prescription, but it appeared that the treatment did not have a positive impact on the condition or survival of the fish. Several moribund fish were sampled at this time and sent to Gribbles Veterinary (VETLAB, Glenside, South Australia, Australia) for pathological testing, which included gross and microscopy pathological examination, microbiology, bacterial swabs, histopathological examinations of the kidney and gills. Pathology results, however, were inconclusive.
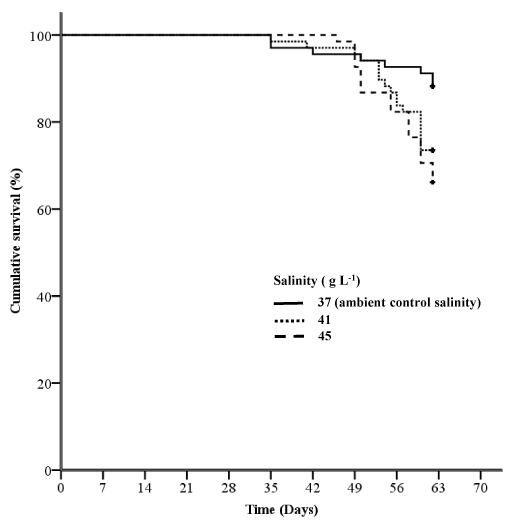
Figure 2 Kaplan-Meier survival curves for fingerling yellowtail kingfish exposed to different salinity levels at 24 °C for 59 days in Experiment 1 (n = 68 for each treatment).
The survival of fingerlings held at the ambient control salinity level of 37 g L-1 was significantly greater than at 41 or 45 g L-1 (Log-rank test, P=0.003; Breslow tests, P=0.004). There was no significant difference in the survival of fingerlings held at salinity levels of 41 or 45 g L-1 (Log-rank test, P=0.366; Breslow tests, P=0.381).
It should be noted that water temperature during experiment 1 (24 °C) was at the upper temperature for optimal growth of YTK.20 We chose this water temperature as it is representative of the temperature experienced by YTK during the fingerling phase of sea-cage production in the Spencer Gulf region. Smaller fish have a higher metabolic rate than the larger juvenile fish;6 this combined with the higher water temperature in Experiment 1, compared to Experiment 2 (20 °C), would have further increased the metabolic rate of fingerlings. This may have resulted in larger proportion of energy in fingerlings being partitioned towards regulating osmo regulation and homeostasis6,16 and may have been a contributing factor to background mortality during Experiment 1. Further research is warranted on the interactive effects of water temperature and salinity on YTK physiology. The life-history of this species is oceanic where juveniles are found in open ocean surface waters in, often associated with flotsam and floating seaweed.5 In Spencer Gulf, where this species is cultured, temperature fluctuates seasonally (10 °C to 24 °C) and spatially, with at least a 3°C difference between north and south of the gulf.1,21
In contrast to the fingerlings in Experiment 1, there were no mortalities in the juvenile YTK in the ambient salinity treatment in Experiment 2 (Figure 3). The survival of juveniles held at salinity levels of 39 g L-1 salinity (ambient) in Experiment 2 was significantly greater than at 41 or 45 g L-1 (Log-rank test and Breslow tests, P = 0.004; Figure 3). There was no significant difference in the survival of juveniles held at salinity levels of 41 or 45 g L-1(Log-rank test, P = 0.690; Breslow tests, P = 0.626). The signs exhibited by juvenile YTK that died in Experiment 2 were similar to those observed in fingerlings in Experiment 1. However, it should be noted that nutrient deficiency may have contributed to background mortalities in both experiments in this study. Prior to 2012, YTK were cultured in Australia using commercial diets based on salmonid feed formulations.22 This is one of the limitations with new species culture; reliance on existing formulations that may not have the nutritional requirements for that species due to limited knowledge. It is now known that YTK health issues prior to 2012 were caused by dietary deficiencies in nutrients such as vitamin E23,24 and taurine.24,25 Australian commercial YTK diets are now formulated with an emphasis on the nutrient requirements of YTK, Japanese yellowtail (Seriola quinqueradiata) and to a much lesser extent, salmonids.
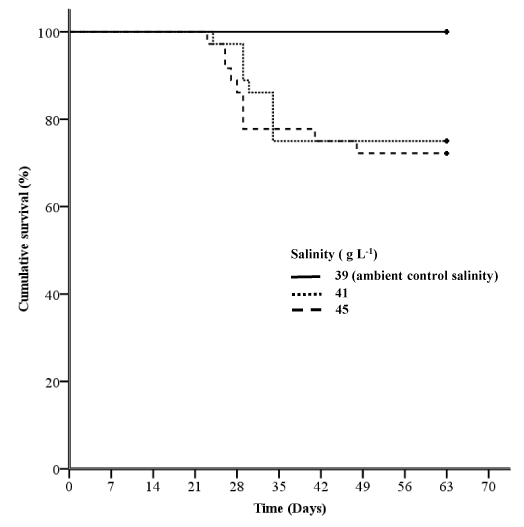
Figure 3 Kaplan-Meier survival curves for juvenile yellowtail kingfish exposed to different salinity levels at 20 °C for 63 days in Experiment 2 (n= 36 for each treatment).
The survival of juveniles held at the ambient control salinity level of 39 g L-1 was significantly greater than at 41 or 45 g L-1 (Log-rank and Breslow tests, P=0.004). There was no significant difference in the survival of juveniles held at salinity levels of 41 or 45 g L-1 (Log-rank test, P=0.690; Breslow test, P=0.626).
Growth Performance
The results for growth performance of YTK from Experiments 1 and 2 are displayed in Tables 1 and 2, respectively. There were no significant differences (P>0.05) in the initial biomass or initial individual weights of fish in each culture unit at the commencement of Experiment 1 or Experiment 2 (Tables 1 and 2). The SGR of the smaller YTK fingerlings grown at 24 °C in Experiment 1 ranged from 3.41 to 4.09 and was ~4 times greater than for larger juvenile fish grown at 20 °C in Experiment 2 (Tables 1 and 2). The FCRs observed for the fingerling YTK grown at 24 °C (Table 1; ranging from 1.28–1.66) were better than observed in the juvenile (Table 2; ranging from 1.81–2.15) fish grown at 20 °C. When compared to data reported for experimentally grown YTK of similar size ranges,18,19 YTK in the current study appeared to grow well and convert feed in an efficient mannerat ambient salinity levels.
In Experiment 1, there were significant reductions in growth performance as measured by weight gain and specific growth rate, observed in fingerling YTK exposed to the 41 and 45 g L-1 salinity levels when compared to the fingerlings cultured at 37 g L-1 (P<0.05; Table 1). In fact, as salinity increased the growth performance of fingerlings decreased significantly between the treatments. Similar trends were observed for growth performance of the larger juvenile fish at lower temperatures in Experiment 2 (Table 2). However, the differences in weight gain and SGR were numerically but not statistically (P>0.05) reduced as salinity levels progressively increased from 39 to 41 to 45 g L-1. These findings are in agreement with the reported reduction in growth rates of juveniles of an extremely euryhaline tilapia species, Sarotherodon melanotheron, when exposed to an increasing salinity gradient ranging from 20 to 118 g L-1 (with increments of 20 g L-1).26-27 Bachman and Rand27 reported adverse effects of acute and gradual salinity changes on fish survival and development for juveniles of four native estuarine marine fish species, Sailfin Molly (Poecilia latipinna), Gold-Spotted Killifish (Floridichthys carpio), Sheepshead Minnow (Cyprinodon variegatus), and Eastern Mosquitofish (Gambusia holbrooki). Conversely, Cotton et al.28 reported that juvenile black sea bass exposed to hypo-saline conditions grew best when salinity was maintained at 20 or 30 g L-1 (more representative of their natural conditions) as opposed to 10 g L-1. Similarly, good growth was reported for ~450 g YTK cultured in diluted (20 g L-1) and normal seawater (37.6 g L-1) at 22 °C.29 This would suggest that there is an optimal range of salinity for growth for YTK and higher levels of ≥ 41 g L-1 appear to be beyond this range. Although being species dependent Boeuf and Payan reported that marine fish generally present higher developmental and growth rates at lower salinity levels (20-30 g L-1) than they normally experience (35g L-1), while freshwater fish do so at higher salinity levels (8-15 g L-1) than they normally experience (<5 g L-1).6 Further research is required to refine the optimal salinity range for YTK growth and survival.
Increasing salinity also had a significant (P<0.05) effect on the fork length of fish in both experiments. For Experiment 1, fork length was significantly reduced at each increasing salinity level (Table 1), while in Experiment 2, fork length was significantly reduced once the salinity level had exceeded 41 g L-1 (Table 1). In contrast, the condition index of YTK was not significantly affected by increasing salinity levels in either experiment. However, there was a trend in both experiments for a numerical reduction in condition index as salinity levels increased (Tables 1 and 2).
Feed utilisation
YTK in both experiments were fed to apparent satiation and the feed intake of fish held at increasing salinities was significantly reduced with increasing salinity levels (P<0.05; Tables 1 and 2). Interestingly, there was also a significant inverse relationship between increasing salinity and FCRs (P<0.05; Table 1). This suggests that fish in both experiments may have been expending considerable energy in an attempt to overcome the possible osmotic imbalance brought about by their failure to adapt to the increasing hyper-saline conditions. Imsland et al.30 reported that both temperature and increasing salinity had a significant effect on growth, daily feed intake and feed conversion efficiency in juvenile turbot, Scophthalmus maximus, with food consumption and food conversion efficiency highest at 18 °C and a salinity level of 15 g L-1, and lowest at 10 °C and a salinity level of 33.5 g L-1.
Osmoregulation
During Experiment 1, the blood plasma osmolality of fingerling YTK was significantly different among treatments at day 1 and 58 (interaction, P<0.01; Figure 4a). YTK fingerlings exposed to the highest salinity (45 g L-1) showed the greatest response, with osmolality significantly decreasing by 24 h, followed by a significant increase by day 59 compared to the ambient salinity (37 g L-1) control group. While fingerlings held at ambient salinity (37 g L-1) exhibited a minor reduction in osmolality by 24 h, they also exhibited a significant increase in osmolality at day 59 compared to the initial levels observed at start of the experiment.
Gill chloride cell abundance was observed to increase in YTK fingerlings exposed to 45 g L-1 (Experiment 1), albeit this trend was not significant compared to ambient salinity control fingerlings held at 37 g L-1 (P>0.05), which may have been attributed to a lack of replicates as suggested by the large error values and low power of the ANOVA test (β=0.25; Figure 4b). SDH activity and Na+/K+ ATPase activity significantly increased in all treatments by day 59 (P<0.01), with no difference observed between ambient salinity control (37 g L-1) and treatment groups (P>0.05; Figure 4c, d). It is worth noting that by the end of Experiment 1 (day 59), mortalities had occurred in both ambient salinity control and treatment groups, and were more prominent at higher salinities (Figure 2).

Figure 4 Blood plasma osmolality (a), gill chloride cell abundance (b), gill SDH activity (c) and gill Na+/K+ ATPase activity (d) of fingerling yellowtail kingfish in response to increasing salinity levels at 24 °C in Experiment 1.
Mean ± standard error of the mean, n = 4. Note that for gill chloride cells, data are not available for the 45 g L-1 treatment on day 1. Letters indicate significant difference from day 0 among pooled day means (on top of data point) or day treatment means (next to data point). Asterisks indicate significant difference among treatments on a given day. All statistical significance is for P<0.05.
During Experiment 2, blood plasma osmolality levels in juvenile YTK did not significantly differ by day 63 (P>0.05), nor did they significantly differ among treatments (P>0.05; Figure 5a). Gill chloride cell abundance significantly increased in juvenile fish exposed to 45 g L-1by day 63 compared to the levels at the start of the experiment (P<0.05; Figure 5b). Similarly, SDH activity significantly increased in juvenile fish exposed to 45 g L-1by day 63 compared to the level at the start of the experiment (P<0.05; Figure 5c). While a similar trend was also observed for Na+/K+ ATPase activity in juvenile YTK, it was not statistically significant likely due to large error values and low power of the ANOVA test (P>0.05; β<0.2; Figure 5d).
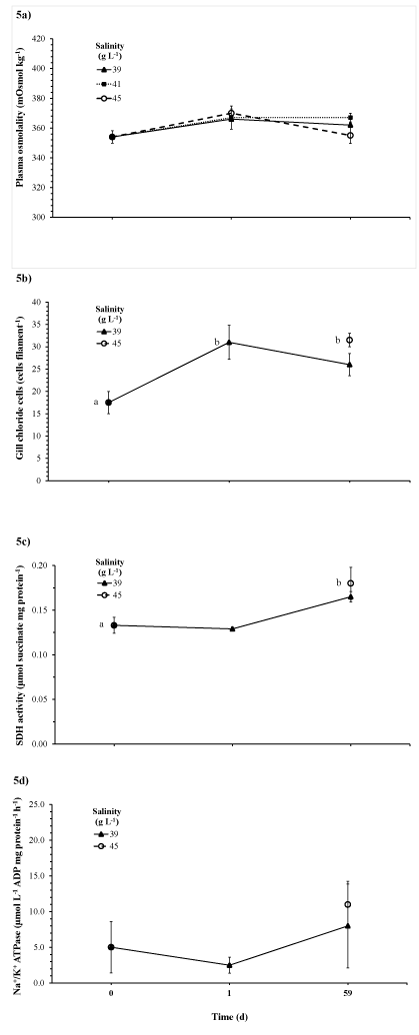
Figure 5 Blood plasma osmolality (a), gill chloride cell abundance (b), gill SDH activity (c) and gill Na+/K+ ATPase activity (d) of juvenile yellowtail kingfish in response to changing salinity levels at 20 °C in Experiment 2.
Mean ± standard error of the mean, n = 4. Note that for gill parameters (Figure b, c and d) data are not available for the 45 g L-1 treatment on day 1. Letters indicate significant difference from day 0 among day treatment means (P<0.05).
Osmolality data suggests that juvenile YTK were more tolerant (able to maintain osmotic homeostasis) to hyper-salinity than fingerling YTK. However, it is unclear whether this was due to age (Experiment 1: fingerlings; Experiment 2: juveniles) or temperature differences (Experiment 1: 24 °C; Experiment 2: 20 °C) or a combination of both. Handeland et al.31 and Imsland et al.13 found that osmoregulatory ability was greater at higher temperatures for Atlantic salmon and turbot, respectively. Generally salinity tolerance in fish increases in the early stages of development with age and size, until during the juvenile phase when osmoregulatory capacity is similar to that of adults.13,32-33 Based on the above observations, and given the younger smaller YTK fingerlings exhibited lower osmoregulatory capacity compared to the older larger juveniles, even at the higher water temperature, it may be more likely that age and size, as opposed to temperature, most influenced the osmoregulatory ability of the YTK during this study. Regardless, this holds implications for site selection and timing of sea-cage stocking and grow-out for YTK aquaculture in South Australia’s hyper-saline gulf systems, and other similar areas. Sites of ambient, more oceanic, salinities would be better for stocking and culture of fingerling and juvenile YTK. Sites in northern Spencer Gulf are characterised by salinities (up to 42 g L-1) and temperatures (up to 25 °C) that are at the upper limits for optimum growth of YTK.20 Sites of higher salinities that are within tolerance ranges (e.g. Arno Bay) may be acceptable during warmer months for larger juvenile or-sub-adults that have better osmoregulatory capability; however, this requires further investigation.
Changes in osmoregulatory parameters of ambient salinity control fish over the experimental period may have been due to stress, disease or a combination of both. This trend was more pronounced in fingerling YTK in Experiment 1, and coincided with mortalities, although close to normal range for laboratory trials,16,18,19 and temperature (24°C) which was at the upper limit of their preferred range for optimum growth.20,34 Osmotic imbalance in fish as a result of stress35,36 and disease12,14,37 is well documented. Varsamos et al.38 showed that husbandry stress (including tank scrubbing and variable water temperatures) during early life stages affected osmolality, body weight and susceptibility to nodavirus in sea bass (Dicentrarchus labrax).
Results from this study suggest that hyper-saline conditions of ≥41 g L-1 at either 24 °C or 20 °C were suboptimal for YTK fingerlings and juveniles performance, respectively. Increasing salinity above ambient levels led to a reduction in growth performance, feed intake, feed efficiency and survival of fingerling and juvenile YTK. These results suggest that fingerling and juvenile YTK do increasingly poorly at salinity levels at and above 41 g L-1 under the conditions tested in both experiments in this study. Osmoregulatory parameters measured in experimental YTK, together with mortality data, suggest that 45 g L-1 is close to the physiological tolerance limitation for this species. Osmolality data suggests that larger juvenile YTK were more tolerant (able to maintain osmotic homeostasis) to hyper-salinity than that smaller fingerlings. This holds implications for site selection and timing of sea-cage stocking and grow-out for YTK aquaculture in South Australia’s hyper-saline gulf systems, and other similar locations. Sites of ambient, more oceanic salinities, would be optimal for both fingerlings and juvenile production. Sites in northern Spencer Gulf are characterised by salinities (up to 42 g L-1) and temperatures (up to 25 °C) that are at the upper tolerance limits for YTK, which are oceanic species. Sites of higher salinities that are within tolerance ranges (e.g. Arno Bay) may be acceptable during warmer months for larger juvenile sub-adults that have better osmoregulatory capability; however, this requires further investigation. In addition, changes in osmoregulatory parameters of ambient salinity control fish over the experimental period may have been due to stress, disease or a combination of both. This trend was more prominent in fingerling YTK, which coincided with mortalities which were close to normal ranges for laboratory trials. It is, therefore, suggested that optimising husbandry techniques in YTK aquaculture to minimise stress prior to and during hatchery to sea-cage transfer, particularly when stocking hyper-saline lease sites, may improve acclimation and performance of stocked fish.
None.
None.

©2014 Stone, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.