Journal of
eISSN: 2572-8466


Short Communication Volume 9 Issue 4
1Departamento de Biotecnología, Universidad Autónoma Metropolitana-Iztapalapa, Mexico
2División de Electromecánica Industrial, Universidad Tecnológica de Tecámac, Mexico
Correspondence: Ernesto Favela-Torres, Departamento de Biotecnología, Universidad Autónoma Metropolitana-Iztapalapa, San Rafael Atlixco 186, Col. Vicentina, Iztapalapa, Mexico City, Mexico
Received: May 12, 2022 | Published: July 7, 2022
Citation: Méndez-González F, Chávez-Escalante G, Loera O, et al. What does respirometric analysis tell us about Metarhizium robertsii. J Appl Biotechnol Bioeng. 2022;9(4):94-96. DOI: 10.15406/jabb.2022.09.00292
Metarhizium comprises species of importance as biological control agents whose conidia have been well produced in lab-scale packed column bioreactors. However, difficulties such as low oxygen supply, CO2 removal, and overheating limit bioreactor scale-up. To select suitable operating conditions in industrial-scale bioreactors, it is necessary to analyze the bioprocess in terms of O2 consumption and CO2 production. Metarhizium robertsii cultures reached maxima of O2 consumption and CO2 production rates of 0.23 and 0.35 mg/gidm h, respectively. The total O2 consumption (23.81 mg/gidm) and CO2 production (34.52 mg/gidm) were determined by experimental measurements and estimates by mathematical models with a coefficient of determination higher than 0.99. The respiratory quotient presented values from 1 to 1.2 (from 48 to 175 h). The respirometric analysis showed that the culture of is an aerobic process with low CO2 and low metabolic heat production, favoring the scale-up of the bioprocess.
Keywords: entomopathogenic fungi, oxygen consumption, carbon dioxide production, packed column, solid culture
Agricultural pests affect several plant species and generate annual economic losses of billions of dollars.1,2 To combat pests, sustainable and free-chemical strategies have been developed. One of these strategies is biological control, which uses various organisms provided with diverse strategies including parasitic, predatory, or pathogenic approaches for reducing pest communities.3 These organisms, called biological control agents, include entomopathogenic fungi of the Metarhizium genus.4 The species of Metarhizium have been widely used because they control a wide range of insect populations5 in diverse climatic conditions.6 For the application in the field, the conidia from entomopathogenic fungi are produced in solid culture using static and stirred bioreactors.4 Those reactors include packed column bioreactors (PCB), used for M. anisopliae and, able to reach higher production and productivity than those obtained in tray and bag bioreactors without reducing the conidia quality.7,8 Therefore, packed columns have been explored to produce Metarhizium conidia on a large scale. Nevertheless, the scale-up of PCB is limited due to poor air distribution, low CO2 and heat removal, and oxygen supply,7,9,10 affecting the fungal culture. These difficulties could be solved by selecting adequate operating conditions and scale-up criteria, which should be based on the microorganism metabolism, monitoring parameters in PCB such as O2 consumption and CO2 production. Both have been used to estimate the growth of the microorganism11,12 and the heat generation9,10 of the culture. Therefore, in this work, a respirometric analysis was carried out in cultures of M. robertsii; and kinetic parameters associated with O2 consumption and CO2 production were mathematically estimated. These parameters can be used to determine the operating conditions for the production of conidia in large-scale PCB.
Microorganism
The used strain of Metharizium robertsii Xoch 8.1 has been deposited into the Culture Collection ENCBIPN
WDCM449 with the identification number ENCB-MG-81. This strain is part of the fungal collection from the Universidad Autonoma Metropolitana-Iztapalapa. The inoculum was produced in Erlenmeyer flasks (250 mL) with 30 mL of Sabouraud dextrose agar (BIOXON, Mexico) and incubated at 30°C for 24 days.10
Bioreactors
Packed column bioreactors (PCB) were used for this work. PCBs were 2 cm inner diameter glass columns with a bed height of 20 cm and a capacity of ~30 g of wet mass (gwm).
Substrate
First-quality rice grains (variety Morelos, Mexico) were used as substrate. The rice grains were raised with tap water and drained until yield 30% of the initial water content remained. Moist rice grains were placed in high-density polypropylene bags, sterilized at 102°C for 15 min, and further stored at 4 °C for 48 hours.8
Inoculum
Conidia produced in Erlenmeyer flask were suspended in 50 mL of sterile Tween 80 solution (0.05% v/v) added with 500 ppm chloramphenicol by stirred with a magnetic bar. Sterilized rice grains were homogeneously inoculated to reach a conidia concentration of 2 x 106 conidia per gram of dry mass (gdm); then, the inoculated substrate was transferred to each PCB as independent experimental units.
Culture conditions
Five PCBs with inoculated substrate were incubated in a temperature-regulated area at 30°C for 175 h. During cultivation, each bioreactor was supplied with saturated air (0.66 L/kgwm min), and O2 consumption and CO2 production were monitored (every 75 min per bioreactor).
Respirometry analysis
The exhaust gas at each PCB output was dried with silica gel and fed to the respirometric analyzer.13 Oxygen consumption and CO2 production rates were obtained from their concentration gradients concerning the air supplied to the bioreactor and expressed as mg per gram of initial dry matter per hour (mg/gidm h). Total O2 consumption and total CO2 production were estimated by assessing the area under O2 consumption and CO2 production rates (respectively) by the trapezoid method.14 Lag time was determined by the intersection of the line formed by LnCO2 production vs time with the x-axis.15 The kinetics associated with CO2 production such as specific rate of CO2 production (µ), initial CO2 production (CO2o), and maximum CO2 production (CO2max) were determined by the logistic model (Equation 1)11 using Generalized Reduced Gradient (GRG) method.16 Maximum O2 consumption (O2max) was estimated by the Soto-Cruz model17 considering O2 as a substrate12 (Equation 2) using the GRG method. For the above, mass yield of CO2 production per O2 consumed (YCO2/O2) and maintained coefficient (mO2) were estimated by multilinear regression. The instantaneous respiratory quotient (CO2/O2) was determined from the molar O2 consumption and CO2 production rates.18
Equation 1 Logistic model.
Equation 2 Modified from Soto-Cruz model.17
Statistical analysis
The correspondence between the data obtained experimentally and those estimated mathematically was evaluated with the coefficient of determination (R2).
To determine the kinetic parameters associated to O2 consumption and CO2 production, their content in the exhaust gas at each PCB output was measured for 165 h. At 100 h of culture, the maximum O2 consumption and CO2 production rates were 0.23 and 0.35 mg/gidm h respectively (Figure 1).

Figure 1 Oxygen consumption (gray) and carbon dioxide production (black) rates in packed column bioreactors.
The Metharizium robertsii culture showed a Lag time of 36.89 h, total O2 consumption of 23.81 mg/gidm, and total CO2 production of 34.52 mg/gidm (Figure 2). The experimental data of CO2 production and O2 consumption were fitted to the logistic and Soto-Cruz17 models respectively (Figure 2). The estimated kinetic parameters correspond to a coefficient of determination greater than 0.99 in both models (Table 1). From 48 to 175 h of culture, the respiratory quotient presented values from 1 to 1.2 (Figure 3).
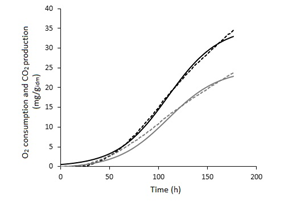
Figure 2 Oxygen consumption (gray) and carbon dioxide production (black). Dashed lines symbolize experimental data and solid lines symbolize mathematically estimated data by the logistic model (black) and Soto-Cruz model (gray).
Logistic model |
Modified Soto-Cruz model |
µ = 0.039/h |
YCO2/O2 = 1.4 mgCO2/mgO2 |
CO2o = 0.51 mg/gidm |
mO2 = 3 x 10 -5 mgO2/mgCO2 h |
CO2max = 35.43 mg/gidm |
O2max = 22.36 mg/gidm |
R2 = 0.997 |
R2 = 0.993 |
Table 1 Mathematically estimated kinetic parameters
The maximum CO2 production rate was attained at 100 h of culture; this is similar to the obtained by M. anisopliae in PCB.18–20 Even under similar culture conditions (inoculum, bioreactor type, aeration, initial substrate moisture, time, and temperature), the values obtained for the maximum CO2 production rate (0.35 mg/gidm h) and the total CO2 production (34.52 mg/gidm) for are comparable to those reported in cultures of M. anisopliae (0.30 mg/gidm h and 35.39 mg/gidm, respectively).8 The similarities between M. anisopliae and suggest that the favorable operating conditions for the cultivation are similar for both species of Metarhizium. The maximum CO2 production rate reached by is up to 55 times lower than that obtained by Aspergillus niger (∼20 mg/gmsi h);21,22 which represents an advantage to avoid metabolic heat accumulation.
The respiratory quotient values obtained during the cultivation of showed that it is an aerobic process that favors the production of biomass,18,23 since the fraction of energy from the organic substrate converted to biomass is ≤0.7.24 Considering the above, the total heat generated by the culture is ∼708 kJ/kgidm. This heat generation is lower than that obtained in other microbial processes in solid culture (>10 000 kJ/kgidm),11 which represents encouraging prospects for scaling up the bioreactor because it favors maintaining the temperature favorable for the culture (30-32 °C).7
The respirometric analysis showed that the culture of using PCB is an aerobic process with low CO2 and metabolic heat production. This could prevent the formation of anaerobic zones and overheating in larger-scale bioreactors. The CO2 production obtained by is like that obtained in Metarhizium anisopliae; therefore, the kinetic parameters evaluated in this work and the bioreactor scale-up criteria obtained from can be helpful to develop bioprocesses for other fungi typically used for biological control.
None.
The authors state that there is no conflict of interest.
None.

©2022 Méndez-González, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.