Journal of
eISSN: 2572-8466


Research Article Volume 10 Issue 1
1Faculty of Agricultural Sciences, Research and Postgraduate Secretariat, National University of Catamarca, Argentina
2Faculty of Agricultural Sciences, National University of the Coast, Argentina
3National Council for Scientific and Technical Research, Argentina
Correspondence: Di Barbaro Gabriela, Faculty of Agricultural Sciences, Research and Postgraduate Secretariat, National University of Catamarca, Argentina
Received: December 29, 2022 | Published: February 2, 2023
Citation: Valeria GB, Gabriela DB, Julieta F, et al. Verticillium dahliae Kleb, regionally important phytopathogen agent. J Appl Biotechnol Bioeng. 2023;10(1):24-30. DOI: 10.15406/jabb.2023.10.00323
Verticillium dahliae is a soil phytopathogenic fungus that presents a wide range of susceptible hosts, both herbaceous and woody, which are considered of agronomic, ornamental and forestry interest. Among the species of agronomic interest and regional importance are the olive tree (Olea europaea) and tomato (Solanum lycopersicum), among others. The application of prevention and control measures requires the identification of the etiological agent that causes diseases, for which the objective of this work was to determine the presence, isolation, and identification of the etiological agent of the Olive Verticillium disease that manifests itself in the olive groves of the provinces of Catamarca and La Rioja, the main olive (Olea europaea L.) producing provinces in the Argentine Republic. Sampling was carried out in different olive farms in Catamarca and La Rioja to obtain the material for the isolation of V. dahliae, until obtaining pure cultures and selection of strains. The morphological and molecular identification of the isolates was carried out. The isolation and morphological, functional, and genetic identification of V. dahliae as a phytopathogenic agent of olive verticillium wilt was achieved, in olive plant var. Arauco of the province of Catamarca.
Keywords: olive verticillium wilt, Olea europea, Catamarca, Argentina
The Verticillium genus corresponds to a soil fungus, hemibiotroph, of great global importance, because it causes damage to crop of economic importance and is taxonomically classified in the Plectosphaerellaceae family.1 This phytopathogen causes yield losses of up to 80 %, making it difficult to control.2 There is no known sexual state, and it has resistance structures called microsclerotia.3 It was found for the first time in Italy by Rugeri in 1946 and later it was identified in different olive-producing countries such as Greece, France, Turkey, among others,4,5 where it caused great damage. Due to the infective capacity and to develop the disease called Verticillium wilt of the Olive Tree.6 This phytopathogen can persist in residues of infected plants through their vegetative structures, conidia and/or mycelium,4 or through the microsclerotia, enabling its perpetration in the soil or in decomposing plant tissue,7 in contrast to the hyphae and conidia that lose their viability in soils in the short term. Verticillium dahliae presents a wide range of susceptible hosts, both herbaceous (including weeds) and woody. These species can be considered of ornamental, forestry, and agronomic interest. These guests can be considered as symptomatic or asymptomatic.8 Among the species of agronomic interest and regional importance are the olive tree (Olea europaea) and tomato (Solanum lycopersicum), among others.
Regarding the predisposing conditions for the growth of V. dahliae, López Escudero and Mercado Blanco3 state that the highest density of inoculum of the phytopathogen is found in humid soils, they are located around drippers of the irrigation system, making the Improper irrigation management may constitute a predisposing factor for the disease, since root growth increases in the humid soil profile, as well as the pathogen. In the same way, nutrition influences the growth of plants and their defense mechanisms against attacks by phytopathogens. Experiences in olive trees indicate that excessive fertilization with nitrogen (N) related to abundant irrigation favors the rapid growth of V. dahliae, which may increase the incidence and severity of infections.3 It was also found that fertilization with N in the form of ammonia (NH3) reduces the number of V. dahliae propagules in the soil and increases the biological activity of pathogen antagonists. Reducing the levels of calcium (Ca) or increasing magnesium (Mg) (for example, through the application of certain organic amendments) in soils, decrease the severity of the disease, while the incidence of potassium (K) about the disease is contradictory, due to the few studies. These effects may be related to different factors such as: soil texture, organic matter content, soil microbiota, among others.3
While the influence of the pH in the soil, in olive verticillium wilt manifests itself at neutral to alkaline pH (pH 7 to 9) and for its control with the application of organic amendments it can become an acidic pH (below 5.5), which is inhibitory. growth of microorganisms and survival structures (microsclerotia) of the pathogen. Likewise, studies show that soils of a saline nature or of induced salinity increase the incidence and severity of wilt diseases in potato, tomato, alfalfa, sugar, and maple.3
The epidemic development of the disease is highly dependent on the agronomic management that is performed on the crop. This telluric fungus remains in infested soils through V. dahliae propagules, such as: spores or conidia, melanized hyphae, and individual microsclerotia or in groups attached to soil particles or plant remains where they are formed; its control being complicated due to the survival capacity of the microsclerotia in the absence of hosts.9,10 The disease begins its development when the propagules have the potential to germinate in the soil (Figure 1) with optimal environmental conditions, such as: temperature, between 20 and 25 °C, soil humidity of 65%,8 and presence of root exudates,9,11 which stimulate the germination of microsclerotia and hyphae emerge and penetrate the roots through wounds, colonize the xylematic vessels forming conidia until reaching the aerial part of the plant, it is not ruled out that the pathogen can also enter the plant through the aerial part.12 This causes damage due to the accumulation of gummy substances that causes the clogging of the vessels and the symptoms of the disease in the plant, causing its deterioration, its senescence is accelerated, and crop production is reduced.3,5,10,12 Dormant microsclerotia are dispersed through movement in the soil or by water, air, irrigation, machinery, etc.3 Once the infected tissues decompose, they release microsclerotia into the soil, which can be incorporated into the soil or through the manure of animals that have fed on remains of diseased plants, remaining as a source of dispersal inoculum, and causing new infections.13,14
The severity of the attacks in olive verticillium wilt depends on the virulence of the V. dahliae pathotype that infects the tree.3 Once the phytopathogen is in the root vascular system, pathogen colonization in the aerial vascular system can be very rapid and eventually reach the leaf petioles. In fact, V. dahliae can progress throughout the entire plant using a cyclical process.15 Wrighy observed the presence of conidia in tobacco plants one hour after inoculation.4 Localized wilting is characteristic of olive verticillium wilt, it is due to the production of toxic substances and accumulated structures in the xylem, product of the presence of the phytopathogen V. dahliae and, in turn, due to physiological changes suffered by the host, such as thickening of the vascular system. As Palanzón and Palanzón16 point out, the symptoms of olive verticillium wilt syndrome depend on the moment of infection, if it occurs at the beginning of the crop, the development of the plants stops and can lead to death, while in more advanced stages Defoliation also occurs, although it could remain a little longer on the trees, which is also linked to climatic conditions, especially temperature.10,17 Eventually some trees can overcome the disease, as a result they produce new tissues around necrotic tissues, previous injuries and branches recovered from affected trees can reach normal yield levels in successive growth periods.
Although Landa del Castillo et al.,18 indicate that the recovery is due to the inactivation of the pathogen in the plant tissue, so that in order to continue the development of the disease, it is necessary for the pathogen to re-infect the tissue, this being accompanied by factors that stimulate the continuity of its cycle and severity of symptoms such as: the susceptibility of the cultivar (olive tree), the density of the inoculum in the soil, race or strain of V. dahliae and environmental conditions.3,4,17 When the infection reaches a certain level, the characteristic symptoms of the olive verticillium wilt disease appear, and the plants may express different symptoms: “slow decline” caused by the V. dahliae strain, Non-Defoliating (of Moderate Virulence) or “rapid decay or apoplexy”, originated by “Defoliant” strains (of Severe Virulence). The first begins in early spring until summer and has the characteristic of presenting symptoms that gradually develop, such as necrosis and subsequent mummification of leaves, flowers, and fruits. The leaves of the affected shoots turn dull green and eventually fall off; while the "rapid decay or apoplexy" occurs at the end of winter beginning of spring and presents buds and dry branches with the presence of purple coloration that advances from the end to the base, the early fall of asymptomatic leaves manifests and sometimes they show the leaves are brown in color and curl up towards the underside, remaining attached to the affected tissue. The growth of the fungus in the xylem tissue takes on purple or brown coloration.3,11,17 Similar symptoms may be due to nutrient deficiencies, root suffocation, or infection by other soil fungi. This disease can cause serious yield losses, as well as the death of the hosts. It was estimated that the yield loss in olive fruits, var. Picual inoculated with V. dahliae, rises between 75% to 89% from the third to the fifth year after sowing in a soil.11 In many hosts, the infection results in reduced growth, with symptoms of epinasty, wilting, leaf chlorosis that can progress to necrosis, darkening of the xylem vessels and leaf abscission.10
The objective of the work was to determine the presence, isolate and identify the etiological agent of the Olive Verticillium disease that manifests itself in the olive groves of the provinces of Catamarca and La Rioja, the main olive-producing provinces (Olea europaea L.) in Argentina.
Sampling and isolation of V. dahliae
In the months of September to November (spring in the southern hemisphere) a total of 25 olive farms were sampled, 13 located in the provinces of Catamarca, in the Departments. del Capital, Valle Viejo, Pomán and Tinogasta and 12 in the province of La Rioja in the department Arauco (Argentine Republic). The plantations had lots of different ages and with different varieties such as: Arauco, Manzanilla Sevillana, Picual, Misión, Barnea, Arbequina and Frantoio. Plots where the plants showed characteristic symptoms of the olive verticillium wilt disease were visited (Figure 2 A,B). The samples were collected following the protocol suggested by González Vera et al.,17 (Figures 2 C, D), and then they were stored in a refrigerator at 6°C. Subsequently, the isolation of V. dahliae was carried out following the methodology recommended by Blanco López et al.,19 and Roca.20 For this reason, twigs 20 to 30 cm long were cut, removing the leaves, and later washed strongly with a sponge and diluted detergent. Then they were disinfected with 2% sodium hypochlorite for 2 min. They were washed with running water for 45-60 min and later with sterile distilled water (Figure 3 A). In a laminar flow chamber, the superficial debarking of the twigs was proceeded with a scalpel. 5 cm segments of plant material (cortex - xylem) were cut and disinfected again with 1 % sodium hypochlorite for 15 seconds, then washed with sterile distilled water and dried on sterile tissue paper. 5 to 6 pieces of plant material were seeded in Petri dishes with sterile potato dextrose agar (ADP) and incubated in the dark at 25 ± 1°C for 14 days (Figures 3 B–E) .

Figure 2 Selection and collection of the olive plant sample: A and B) olive tree with sectoral necrosis; C) branch with leaves with apical chlorosis; D) Cross section of olive branches with the presence of brown internal tissue.

Figure 3 Disinfection and sowing of the olive plant sample. A: disinfection of twigs, with sodium hypochlorite; B: superficial debarking of twigs; C: peeled tissue (cortex - xylem) and D: superficial disinfection of plant material to be planted. E: seeding of plant material in ADP.
Obtaining pure cultures and selection of strains
To ensure that a pure culture was being used, isolations were performed by surface depletion, streaks were sown in ADP culture medium from the colonies identified as V. dahliae. They were incubated under dark conditions at 25 ±1 °C for 7 to 10 days. Then, with the help of an Olympus SZ 11-CTV stereoscopic microscope, the characteristics of the colony were observed and later with an Olympus BH-2 A:40X optical microscope, these identifications were confirmed (Table 1).
V. dahliae |
Asexual reproduction |
Table 1 Morphological characteristics of V. dahliae
Morphological identification and strain conservation
The isolated strains of the phytopathogenic V. dahliae were morphologically identified at the genus level by means of macroscopic observations of the colony and microscopic observations using the Olympus BH-2 optical microscope with 10 and 40X magnification. The fungal material was colored with cotton blue and exposed to different measurements following the technique of French and Herbert. The strain was preserved in peak flute test tubes with ADP at 5°C. The isolates obtained were incorporated into the strain collection of the Chair of Phytopathology, Fac. de Cs. Agrarian – UNCa (Table 2).
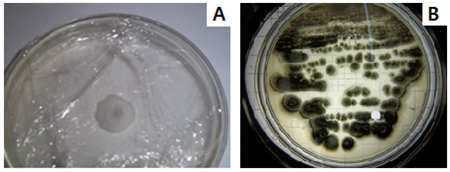
Figure 4 V. dahliae colonies on ADP A: cottony white mycelium, B: colonies of stellate microsclerotia.

Figure 5 Sampled olive field. Collection of samples with verticillium wilt symptoms, from which the isolated strain was obtained.
Microsclerotia |
Size (length / width ratio) |
From highly virulent isolates |
4.06 (µ) – elongated |
From slightly virulent isolates |
2.92 (µ) – rounded |
Table 2 Characteristics of V. dahliae microsclerotia according to the type of isolate
Molecular identification
The isolated strain of V. dahliae was sent to the Macrogen – Korea laboratory, where the genetic material was extracted, the ITS1 – ITS4 regions amplified, with the primer: ITS1: 5´ (TCC CTA GGT GAA CCT GCT G) 3' and ITS4 5' (TCC TCC GCT TAT TGA TAT GC) 3'.2 For sequencing, the kit was used: Big Dye terminator cycle sequencing Kit v 3.1, in the Applied Biosystems model 3730XL sequencer, Macrogen automated DNA sequencing system. With these results, molecular identification was carried out, by means of comparisons of the sequences obtained with the GenBanK database, specifically taking as reference the rRNA/ITS database for fungi, which contains curated and reannotated records of the sequences of the ITS region specific to the Fungi group. The sequenced sample was identified with the help of the BlastN Report software. In addition, the percentage similarity of the sequences obtained with the standard strain was calculated, using tools available on the NCBI (National Center for Biotechnology Information) website.
Sampling and isolation of V. dahliae
From the sampling carried out on farms in the province of Catamarca, the presence of V. dahliae was obtained in nine (9), while in six (6) farms in the province of La Rioja (Table 3). The total number of plant samples collected and processed were 94, of which in 25 samples it was possible to isolate the phytopathogen of interest. The symptomatology observed in the collected samples was sectorized necrosis of secondary branches, where the leaves presented abaxial curling and leaves with apical chlorosis accompanied by slight defoliation. In a cross section of the collected twigs, light brown internal tissue corresponding to the xylem vessels was observed (Figure 2 D). The incidence and severity of the disease and the characteristics of the isolated colony were also considered. The varieties that were verified as hosts and positive for the presence of V. dahliae were Arauco, Arbequina, Manzanilla, Picual and Frantoio. In the remaining samples, some failed to isolate V. dahliae, while others were discarded due to heavy contamination. In the same way as the positive ones, the presence of bacterial and fungal contaminants was observed. The genera of the predominant contaminating fungal agents were Fusarium spp., Alternaria spp., Rhizopus spp., Penicillium spp. and Aspergillus spp.
Sample |
Plant |
Coordinates |
Variety |
Lot |
Observations |
Sampling |
1 |
1 |
S 28° 55.342 |
Picual |
P5 |
Turkey eye, sooty mold, eriophids, anthracnose |
Soil and aerial part |
2 |
1 |
S 28°54´64,3 |
Picual |
A5 |
Plant with dry, necrotic sectors |
aerial part |
3 |
2 |
S 28° 55.910 |
Picual |
P4 |
Completely dry with regrowth |
Soils, aerial part and leaf litter with white mycelium |
4 |
2 |
S 28°55´301 |
Picual |
P5 |
Plant with dry, necrotic sectors |
aerial part |
5 |
3 |
S 28° 55.979 |
Picual |
P4 |
Dry plant |
Photo |
6 |
4 |
S 28° 55.975 |
Picual |
P4 |
extracted plant stump |
Soil |
7 |
5 |
S 28° 55.974 |
Picual |
P4 |
Dry plant with regrowth |
aerial part |
8 |
5 |
S28°55´95.30 |
Picual |
A3 |
Plant with dry, necrotic sectors |
aerial part |
9 |
6 |
S 28° 55.962 |
Picual |
P4 |
Dry plant with regrowth |
Soil and aerial part |
10 |
7 |
S 28° 54.651 |
Barnea |
A3 |
Symptoms of tuberculosis, galls |
photo |
11 |
10 |
S 28° 55.48´´ |
Arauco |
A5 |
Cup-shaped pruned plant, frost |
Soil and aerial part |
12 |
9 |
S 28° 55.780 |
Arbequina |
A8 |
plant with dry branches |
Soil and aerial part |
13 |
10 |
S 28° 54.639 |
Barnea |
A3 |
Symptoms of tuberculosis, galls |
Soil |
14 |
9 |
S 28° 54.653 |
Barnea |
A3 |
plant with dry branches |
Soil and aerial part |
Table 3 Sampling carried out at Agropecuaria Arphi S.A, in the months of September - October in the province of Catamarca
Obtaining pure cultures and selection of strains
It was possible to obtain several isolates of V. dahliae where the characteristics of the colonies were observed. The pure culture from a single colony developed on ADP, if on the surface of this medium each colony has developed from the germination of an individual microsclerotia from the sample. The colonies of microsclerotia obtained presented a stellate appearance with a regular distribution, bright dark coloration, with the presence of exudates (Figure 4 B), cottony white mycelium and defined growth (Figure 4 A). In addition, hyaline and septate mycelium was observed under a microscope. However, the choice of the strain was made considering, among other things, the symptomatology that the plant presented, characteristic of verticillium wilt of the olive tree. Therefore, we worked with the strain obtained in the Department of Capayán, province of Catamarca, from the Arauco olive variety, georeferenced at 28°55¨20.48''S, 65°46'4.98''W (Figure 5 & Table 3).
Characterization and morphological identification
The colonies observed after 10 days of incubation obtained 7cm ±2 in diameter. The observed microsclerotia colonies were of regular distribution (Figure 6), as was the size of the microsclerotia, with a spherical shape predominating over some more flattened ones and forming dense, dark brown, melanized masses with small droplets of exudate (Figures 4). The measurements obtained from the different parts of V. dahliae are presented in Table 4.
Part of V. dahliae |
in microns (µ) |
Mycelium |
Diameter: 0,96 |
Phialides |
Long: 5,76 – 9,6 /Broad: 0,96 |
Microsclerotia |
Diameter: 16,3 |
Conidia |
Diameter: 10,32 |
Chlamydospores |
Diameter:1,92 |
Table 4 Microscopic measurements of V. dahliae A:40X
The whitish aerial mycelium, abundant, dense, with some drops of exudate. Under the microscope, the hyphae are observed hyaline, septate, with smooth walls. The phialides (conidiophores: cf) of 3 or more, form whorls, being narrower at the apex, are hyaline, arranged erect or inclined. The conidia are also hyaline with smooth walls, unicellular and on the phialides are found in groups of 3 or more, rounded or oval (Figure 7 A).

Figure 7 Photomicrograph of V. dahliae: A: fruiting, A:40X (cf=conidiophores, f=phialides, c=conidia, m=mycelium). B: colony of microsclerotia, A: 2.0 X. C: microsclerotia, A: 10 and 40X.
Molecular Identification
The native strain isolated as a phytopathogen was identified as V. dahliae Kleb. The strain shows a 99% identification percentage with respect to the databases provided by the NCBI and a 68% similarity percentage with the standard strain (Genbank: MW229266).
Verticillium dahliae presents mycelium with conidiophores or mononuclear and hyaline phialides as well as ovoid-shaped conidia that originate from the tip of the phialides, the latter are arranged forming erect whorls.3,9 This phytopathogen has two phases: the parasitic, from the infection of the plant or host and the non-parasitic, where the microsclerotia originate,3 which are resistance structures of variable shape and size. (15-20 μ in diameter),4 dark in color, melanized, which gives it the ability to survive for around 14 years, being able to withstand different adverse (Table 1).3,11
The difference between the microsclerotia produced by highly virulent isolates is that they are elongated, presenting an average length/width ratio of 4.06 micrometers, while those produced by slightly virulent isolates were more rounded and showed an average ratio of 2.92 micrometers (Table 2).3 The time of year that the plant material in olive plants was sampled coincides with what other authors state,21,22 since it is the most favorable time for the development of the fungus, since in the extreme T° the phytopathogen is inactivated.
The methodology used allowed the isolation of V. dahliae, for which work was done to adjust the technique to minimize the contamination of the accompanying microflora. The symptoms found in infected plants agree with those described by other authors,3,23–25 where stroke is an early death of the plant, the leaves curl, without having marked defoliation and necrosis prevails,23 and on the other hand the slow decay keeps the dead leaves on its branches, these are generated at the end of winter - beginning of spring and during spring to early summer respectively.3,25 In turn, the internal darkening that was observed in the stem is like that found by León Ttaca et al.,2 in a cross section of the root while in the stem they displayed black scores.
The symptoms presented by infected plants depend on the degree of resistance or susceptibility of the olive varieties and their behavior against the Defoliating (D) or Non-Defoliating (ND) pathotype of V. dahliae that colonizes it. The inoculum density is important in the soil and the edaphoclimatic conditions26 and the agronomic management.27 The olive varieties that were positive for achieving the isolation of the phytopathogen of interest, V. dahliae, were: Arauco, Manzanilla, Picual and Frantoio. Although all the sampled plants presented characteristic symptoms of the disease, in some cases more markedly than in others, the results obtained do not agree with previous studies, where the Frantoio variety is classified as resistant together with Chanlot Real and Empeltre, in the case on the contrary, that of Manzanilla de Sevilla and Picual, which are classified as susceptible3 as well as.28 While López Escudero et al.,23 characterized the most economically important cultivars such as Cornicabra, Manzanilla de Sevilla, Arbequina, Picual and Hojiblanca susceptible or extremely susceptible to one or both pathotypes D and ND of V. dahliae. Therefore, the isolation obtained from var. Frantoio is relevant data, considering that it could be a long-standing infection, where the plant could not recover or, as stated by Trapero et al.,6 about the great variability, the little consistency of the observations, as well as the lack of information on the existing inoculum in the soil and the authenticity of the evaluated cultivars that prevent validating results in the field. Trapero et al.,6 refer to the fact that resistant varieties have the ability to restrict the growth of the phytopathogen in the plant, thus generating a delay in the onset of disease symptoms, as well as a better recovery of infected plants and less percentage of dead plants with respect to susceptible varieties.23
The characteristics of the strains under study coincide with the results obtained by other authors.2,29 However, the measurements obtained from the different morphological parts do not agree with the results obtained by Inderbitzin et al.,29 and Jiménez Díaz et al.30 The morphological characteristics of the microsclerotia allow them to be related to the virulence of the isolates (D and ND). Therefore, evaluations in Agar Agua culture medium of pathotype D produced colonies with mixtures of elongated, rounded and only rounded microsclerotia in non-defoliant isolates.23 In addition,24 states that the composition of the culture medium seems to influence the morphology of the microsclerotia as it serves to establish the isolated pathotype as a preliminary study; however, this must be verified with biological or molecular tests. Lopez Escudero et al.,24 in APM (Modified Sodium Polypeptane Agar) observed that isolate D produces colonies of greater diameter than ND with stellate shapes and elongated microsclerotia, while in ND they are more rounded. According to the characteristics studied by the different authors and based on the results obtained in our study, it is estimated that the isolate under study is an ND strain, considering the size of the microsclerotia that is like that described for this strain. and the symptoms observed in the host, which agrees with what has been published by other authors22,31 who state that it is the only strain found in Argentina. Likewise, isolation through the microbiological method is less effective since this pathotype is found in a lower concentration in the plant compared to D.22 Therefore, what was stated by López Escudero and collaborators32 is considered valid, that there is a coexistence of different V. dahliae, genetic groups of virulence in the same field and even in the same infected tree. The regularity of the microsclerotia was also considered, calling them homogeneous, or irregular in which projections or short melanized cell groups are present that join the body of the microsclerotia with the hyaline mycelium.32
To verify the identity of the phytopathogen, molecular techniques were used due to their effectiveness compared to the classical microbiological technique.21 For this, the ITS 1 / ITS 4 primers were selected, which allowed us to define the genus and species of the phytopathogen in accordance with what was used by León Ttaca et al.2 Other authors used specific primers for the identification of V. dahliae VDFE1/VDFE234 and NDf / NDr, INTNDf / INTNDr and INTND2f / INTND2r,33 using the latter to define the pathotype. Regarding the comparison of sequences, there was a difference with respect to the homology obtained by León Ttaca et al.,2 probably due to the different methods used.
The isolation and morphological, functional, and genetic identification of V. dahliae as a phytopathogenic agent of olive verticillium wilt was achieved, in olive plant var. Arauco of the province of Catamarca, Argentine Republic.
None.
There are no conflicting interests declared by the authors.

©2023 Valeria, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.