Journal of
eISSN: 2572-8466


Research Article Volume 9 Issue 2
1Laboratorio de Transducción de Señales, Instituto de Investigaciones Químico-Biológicas de la Universidad Michoacana de San Nicolás de Hidalgo, México
2Tecnológico de Monterrey, School of Engineering and Sciences, México
3School of the Environment, Florida A&M University, United States of America
Correspondence: Elda Beltran-Pena, Laboratorio de Transducción de Señales, Edificio B3, Instituto de Investigaciones Químico-Biológicas de la Universidad Michoacana de San Nicolás de Hidalgo. Ciudad Universitaria. Francisco J. Múgica s/n, C.P. 58030, Morelia, Michoacán, México
Received: February 12, 2022 | Published: March 7, 2022
Citation: : Carrillo-Flores E, Arreola-Rivera J, Pazos-Solís DM, et al. TOR participation on the root system changes of Arabidopsis during its interaction with Azospirillum. J Appl Biotechnol Bioeng. 2022;9(2):18-23 DOI: 10.15406/jabb.2022.09.00280
The root system of the plant is essential for taking up water and nutrients, serves as an anchor and is the organ where plant-microorganism interaction takes place. When the Plant Growth Promoting Rhizobacteria (PGPR) Azospirillum brasilense Sp245 colonizes the root of the plants, it halts the growth of the primary root and stimulates the development of the lateral roots and root hairs which support vegetative, green biomass. Target of Rapamycin (TOR) is a highly conserved protein in all eukaryotes, and it controls anabolic processes, such as cell cycle, ribosome biogenesis, protein synthesis, cell wall changes and photosynthesis among others. TOR in plants forms part of the TORC1 complex, which when is activated by auxins and light, activates anabolic processes and represses autophagy. TOR regulates the growth of the primary root of Arabidopsis through cell proliferation and elongation. In the present investigation, the participation of TOR during the Arabidopsis-Azospirillum interaction was determined using two approaches, a pharmacology and other genetic. The results showed that TOR is involved in the development of the lateral roots of A. thaliana seedlings inoculated with A. brasilense.
Keywords: Arabidopsis, Azospirillum, TOR, auxins, PAT
PGPR, plant growth promoting rhizobacteria; TOR, target of rapamycin; PAT, polar auxin transport
The Target of Rapamycin (TOR) is an evolutionarily conserved kinase from yeasts, mammals and even plants, that integrates energy and nutrients to promote cell proliferation and growth. In metazoans and mammals, TOR is part of two different protein complexes: TORC1; mTOR (mammalian TOR), RAPTOR (Regulatory-Associated Protein of mTOR) and LST8 (Lethal with SEC13 protein 8) and TORC2; RICTOR (Rapamycin-Insensitive Companion of mTOR), SIN1 (SAPK-Interacting Protein 1) and LST8. TORC1 is part of the nutrient-sensitive pathway that regulates protein synthesis and growth, while TORC2 is activated by growth factors and regulates cytoskeleton rearrangement.1 It is important to point out that TORC1 is the rapamycin-sensitive complex and is the only one that has been reported in plants,2–4 where it is activated by auxins, abscisic acid, light and glucose.5 The reported plant resistance to rapamycin and the embryo mutant lethal of the Arabidopsis to have hindered functional dissection of TOR signaling in plants.6,7 However, Xiong and Sheen8 showed that Arabidopsis grown in liquid medium is sensitive to rapamycin. On the other hand, Xiong et al.,9 generated estradiol-inducible tor transgenic Arabidopsis plant (tor-es1) to circumvent the embryo lethality of null tor mutant. Currently, it has been reported that second-generation TOR inhibitors that compete for the ATP-binding site of TOR, such as AZD-8055, Torin1 and Torin2, among others, alter plant growth.9–12
On the other hand, which belongs to the α-proteobacteria subclass, is capable of improving the growth, development and productivity of more than a hundred plant species through root colonization, causing an increase in the seed production of crops with commercial interest therefore it has become one of the most studied PGPR.13–15 To date, the observed effect on root architecture of most of the PGPRs analyzed including Azospirillum, has been a reduction in primary root growth and an increase in the number and length of lateral roots, in addition to stimulating the elongation of root hairs. The effect of Azospirillum on plant development has been attributed to the phytohormones it produces, mainly auxins.16–19
Auxins are plant growth regulators that participate in practically all plant development processes from reproduction, embryogenesis, hypocotyl elongation, formation of lateral roots and root hairs, besides of regular tropic responses to light and gravity.20 Indole-3-acetic acid (IAA), is the natural auxin that is synthesized in the young shoots of the aerial part of the plant, from where it is distributed to the demanding tissues by two types of transport; one fast and non-directional through the phloem and the other directional that is carried out cell with the help of influx and efflux carriers; it is known as polar auxin transport (PAT).21
Recent reports have shown that TOR participates in the physiological and morphological changes that plants present when interact with microorganism. For example, Nanjareddy et al.,22 observed that TOR is involved during the interaction of Rhizobium with bean roots. It has also been reported that a decrease in TOR expression confers at plant resistance to Fusarium graminearum in Arabidopsis.23 For their part, Sun et al.,24 observed that TOR signaling controls the infection in rice by the fungus M. orizae in rice. Due to the aforementioned, the objective of the present study is to investigate the participation of TOR on the alteration of root architecture during the Arabidopsis-Azospirillum interaction.
Disinfection, sowing and incubation of seeds
The seeds of Arabidopsis Ecotype Columbia (Col-0), transgenic lines DR5::uidA,25 CyCB1::uidA,26 Exp7::uidA27 and the estradiol-inducible tor mutant (tor-es1)8 were disinfected with a 5% chlorine, 1% SDS solution and stratified at 4°C for 48 h. Subsequently, the seeds were sown in Petri dishes containing 0.2X Murashige and Skoog (MS) culture medium, 0.5% sucrose, 0.8% agar and 200 μL/L of a vitamin solution (thiamine 1 mg/mL, pyridoxine 5 mg/mL and nicotinic acid 5 mg/mL) and incubated in a vertical position for 5 days in a plant growth chamber (Percival Scientific AR-95L, Parry, IA, USA), with a photoperiod of 16 h light/8 h dark at 22 °C and a light intensity of 100 µmol m-2 s-1.
Preparation of culture medium
The Murashige-Skoog (MS) culture medium (Phytotechnology Laboratories) was prepared by dissolving in distilled water the amount of salts corresponding to a 0.2X concentration and 0.5% of sucrose. After adjusting the pH to 5.7, 0.8% agar was added. Thereafter, 200 µL/L of a vitamin solution was added to the warm medium previously sterilized. All reagents used came from the Sigma brand unless otherwise specified.
Preparation of culture medium supplemented with different chemical inhibitors
The following compounds dissolved in DMSO were added to the warm MS culture medium previously sterilized: 1 µM N-1-naphthylphthalamic acid (NPA) and 1000 nM AZD-8055 (LC Laboratories). In the experiments with the estradiol-inducible tor mutant (tor-es1), 10 µM estradiol was added to the culture medium.
Preparation of culture medium for maintenance and inoculation of Azospirillum brasilense Sp245
The bacteria were kept in Luria-Bartani (LB)-tetracycline culture medium (10 g/L peptone, 5 g/L yeast extract, 5 g/L NaCl, 0.186 g/L MgSO4, 0.2775 g/L CaCl2, 1 g/L agar and 10 µg/mL tetracycline) at 4 °C; it was reseeded every 10 days in Petri dishes with LB-tetracycline medium, and incubated overnight at 37 °C. Then the bacterium was incubated for 16 h at 37 °C, afterwards an aliquot of bacteria was taken from this plate and resuspended in 1 mL of sterile distilled water to reach absorbances of 0.9 equivalent to 1x108 CFU/mL or 2.5x105 CFU which was added to the medium 0.2X MS, 0.5% sucrose, 0.8 % of agar previously sterilized and warm.
Transfer of seedlings to culture media supplemented with Azospirillum brasilense Sp245 and different inhibitors
5 day-old seedlings were transferred to Petri dishes containing 0.2X MS medium with 2.5X105 CFU/mL of A. brasilense or without the bacterium, and at culture mediums supplemented with the 1 µM NPA or 1000 nM AZD-8055. The seeded Petri dish were incubated for one, three, and six days in the plant growth chamber under the aforementioned conditions.
Histochemical activity of uidA
Transgenic seedlings expressing DR5::uidA, CycB1::uidA y EXP7::uidA were exposed to the bacterium for 1, 3 and 6 days. The seedlings were placed in microtiter boxes, and incubated in a solution of X-Gluc (5-bromo-4- chloro-3-indolyl-β-D glucuronide) (Phytotechnology Laboratories) (1 mM in a phosphate buffer (NaH2PO4 and 0.1 M Na2HPO4 pH 7) adding 2 mM of K4Fe (CN)6 and K3Fe (CN)6, at 37 ºC for 16 h.28 The seedlings were then incubated in an acid solution (0.24 N HCl, 20% methanol) for 80 min at 60 °C, transferred into in a basic solution (7% NaOH, 60% EtOH) for 40 min at room temperature and dehydrated with 40%, 20% and 10% ethanol solutions for 15 min.28 Finally, 50% glycerol was added and the seedlings were incubated overnight. The seedlings were mounted on slides and observed under the microscope with a 4X objective; photographs of the primary root and lateral roots were taken.
Evaluation of root architecture of seedlings
In the 6, 8, 11 and 14 day-old seedlings, corresponding to 1, 3, 6 and 9 days of exposure to bacteria, the parameters of the root architecture were determined: Primary Root Length (PRL), Lateral Root Number (LRN) and Lateral Roots Density (LRD). The PRL was measured with a ruler (cm), the LRN was obtained by counting the roots emerging from the primary root under a stereomicroscope (Iroscope ES-24 Leica) with a 4X objective and the LRD was obtained by calculating the ratio between LRN and PRL.
Evaluation of lateral roots length
The Lateral Root Length (LRL) was determined by photographs of the seedlings with a close-up in the primary root differentiation zone, and then the LRL (cm) was measured with the Image J program version 1.52.
Statistical analysis
All the experiments were carried out at least three times independently, taking 25 seedlings per treatment as a representative sample. Statistical analysis was applied to the data obtained, with the Statistic 8.0 program, and the data were analyzed by univariate with the post hoc Tukey test with a statistical significance of (p <0.05).
Azospirillum alters Arabidopsis root architecture
In this study, the changes of Arabidopsis root system at short times of exposure to the bacteria were analyzed. To evaluated the effect of Azospirillum on root architecture, 5 day-old Wt seedlings were transferred to culture media without and supplemented with 2.5x105 CFU/mL of A. brasilense and incubated for 1, 3 and 6 days. In Figure 1A, it can see that Azospirillum inhibited the growth of root primary throughout all incubation times, while the lateral roots number increased after three and six days of exposure to the bacteria (Figure 1B) and the lateral roots density increased twenty-five times (Figure 1C). Representative images of seedlings clearly show that Azospirillum induces a reprogramming of root architecture from the third day of exposure to the bacteria, in which auxins could be involved (Figure 1D). Due to that this phenotype has been attributed to high concentrations of auxins,29 we analyzed the level of auxins in 5 day-old DR5::uidA seedlings exposed to Azospirillum for one, two, three, four, five, six and nine days. As can be seen in the Figure 1E, on day three of interaction, the level of auxins increased in primary root vascular tissue, particularly, in the differentiation zone of lateral roots; this effect intensified by day fourth of exposition, while that the maximum of auxins in primary root meristem disappear from fifth day of interaction with the bacteria and the auxins accumulated in vascular tissue are redistributed to lateral root meristems.
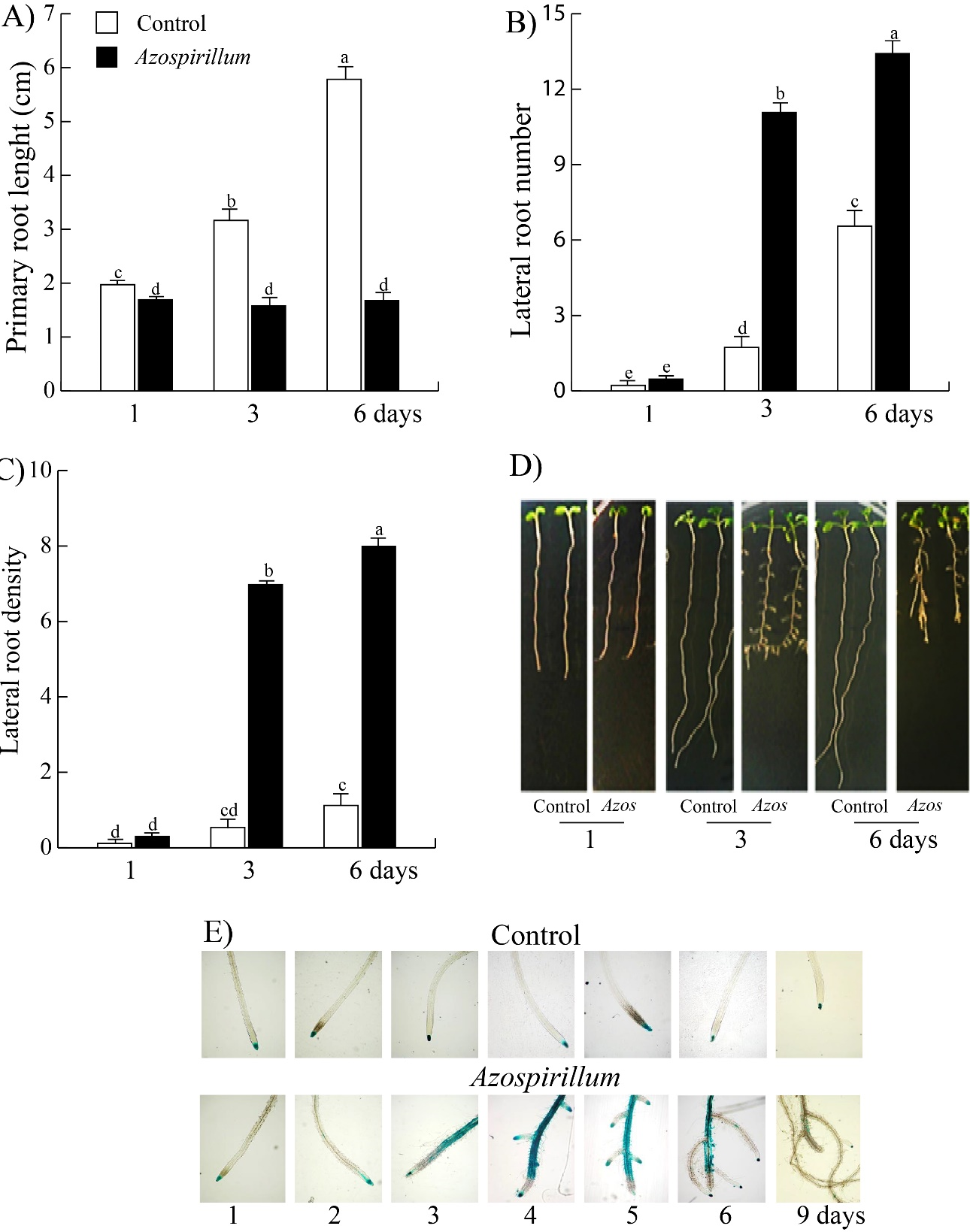
Figure 1 Effect of Azospirillum brasilense Sp245 on root architecture and auxin levels in roots of Arabidopsis. Arabidopsis Wt seedlings were transferred to control media and supplemented with 2.5x10⁵ CFU/mL of Azospirillum, and incubated by 1, 3 and 6 days. A) Primary root length. B) Lateral roots number. C) Lateral roots density. D) Representative photographs of seedlings grown in control medium and inoculated with 2.5x105 CFU/mL of A. brasilense. Values represent the mean of thirty seedlings +/- standard error. The letters indicate the statistically significant differences between the parameters of the Tukey test (P≤0.05). The experiments were repeated in triplicate with similar results. E) DR5::uidA seedlings were transferred to control media and supplemented with 2.5x10⁵ CFU/mL of Azospirillum and incubated by 1, 2, 3, 4, 5, 6 and 9 days. At the end of incubation time, the seedlings were treated with X-Gluc, followed by a clearing process. The seedlings were mounted on slides and microscopic observations were made at 4X magnification. Representative photographs of the roots of the seedlings in the control medium and inoculated with Azospirillum of at least 25 seedlings from three different trials with similar results are shown.
Participation of polar transport of auxins on the root system of Arabidopsis exposed to Azospirillum
Respect to the changes observed in the auxin levels in the primary root and its distribution to lateral roots in Arabidopsis when interacting with Azospirillum (Figure 1E), its polar transport (PAT) could be modulated. As N-1-naphthylthalamic acid (NPA) is a specific inhibitor of PAT,30,31 we evaluated the effect of NPA over changes in root architecture in presence of the bacteria. For this, Wt seedlings were transferred to culture media with 1 µM NPA to assess the interaction with Azospirillum. Subsequently, the material was incubated for 3, 6 and 9 days under the aforementioned conditions. The Wt seedlings grown in the presence of Azospirillum and Azospirillum plus NPA, had a decrease in root length at different times of exposure (Figure 2A), while lateral root number in the presence of Azospirillum and NPA increased in a lesser proportion than in seedlings treated only with the bacterium (Figure 2B). These results show that PAT inhibition prevents the stimulation of lateral root development by the bacteria.

Figure 2 Effect of PAT inhibition on the root architecture of Arabidopsis seedlings exposed to Azospirillum. Arabidopsis Wt seedlings were transferred to control media and supplemented with 2.5x10⁵ CFU/mL of Azospirillum, added both media with NPA (1 µM) and incubated by 3, 6 and 9 days. A) Primary root length. B) Lateral roots number. C) Lateral roots density. D) Representative photographs of seedlings. Values represent the mean of thirty seedlings +/- standard error. The letters indicate the statistically significant differences between the parameters of the Tukey test (P≤0.05). The experiments were repeated in triplicate with similar results.
Participation of TOR on the root system changes of Arabidopsis induced by Azospirillum
It has been reported that the primary root growth depends on TOR through the regulation of cell proliferation and elongation.10,32 To analyze if the changes in the root architecture of Arabidopsis caused by the interaction with Azospirillum depend on TOR, two experimental approaches were used. In the first, the effect of inhibition of TOR with AZD-805512 was tested. For this, Wt seedlings were transferred to culture media with 1000 nM AZD-8055 to assess the interaction with Azospirillum. Subsequently, the material was incubated for 1, 3 and 6 days under the aforementioned conditions. A similar reduction in roots length was observed in all treatments, while the root branching stimulated by the bacteria decreased on the third and sixth day by 52 and 36% respectively, in presence of AZD-8055 (Figures 3(A&B)). On the other hand, the lateral roots length was reduced by 80 and 45% in the seedlings exposed for three and six days respectively to Azospirillum plus AZD-8055 compared to the effect caused only by the bacteria (Figure 3D).
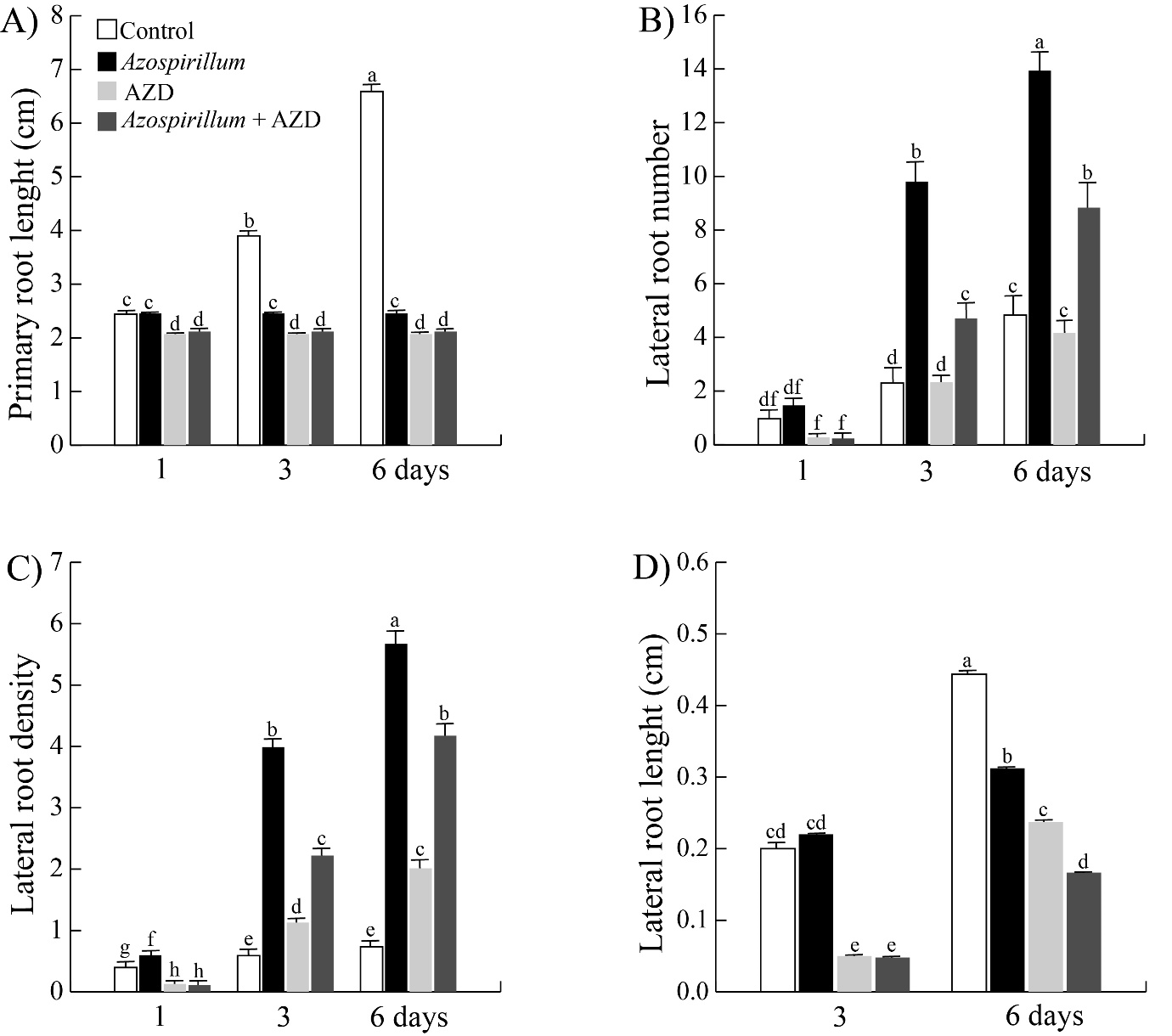
Figure 3 Effect of TOR inactivation on the root architecture of Arabidopsis seedlings exposed to A. brasilense. Arabidopsis Wt seedlings were transferred to control media and supplemented with 2.5x10⁵ CFU/mL of Azospirillum, added both media with AZD-8055 (1000 nM) and incubated by 1, 3 and 6 days. A) Primary root length. B) Lateral roots number. C) Lateral roots density. D) Lateral roots length. Values represent the mean of thirty seedlings +/- standard error. The letters indicate the statistically significant differences between the treatments with a Tukey test (P≤0.05). The experiments were repeated in triplicate with similar results.
For the genetic strategy the estradiol-inducible tor mutant (tor-es1) seedlings, were transferred to culture media with 10 µM estradiol to assess the interaction with Azospirillum. Subsequently, the material was incubated for 1, 3 and 6 days under the aforementioned conditions. A similar reduction in roots length was observed in all treatments, while the lateral roots number stimulated by the bacteria decreased on the third and sixth day by 45 and 33% respectively in presence of estradiol (Figures 4(A&B)). Moreover, the lateral roots length was reduced by 38 and 50% in the seedlings exposed for three and six days respectively, to Azospirillum plus estradiol compared to the effect caused only by the bacteria (Figure 4D). Thus, the results of both strategies suggest that the development of Arabidopsis lateral roots during their interaction with Azospirillum depends on TOR.
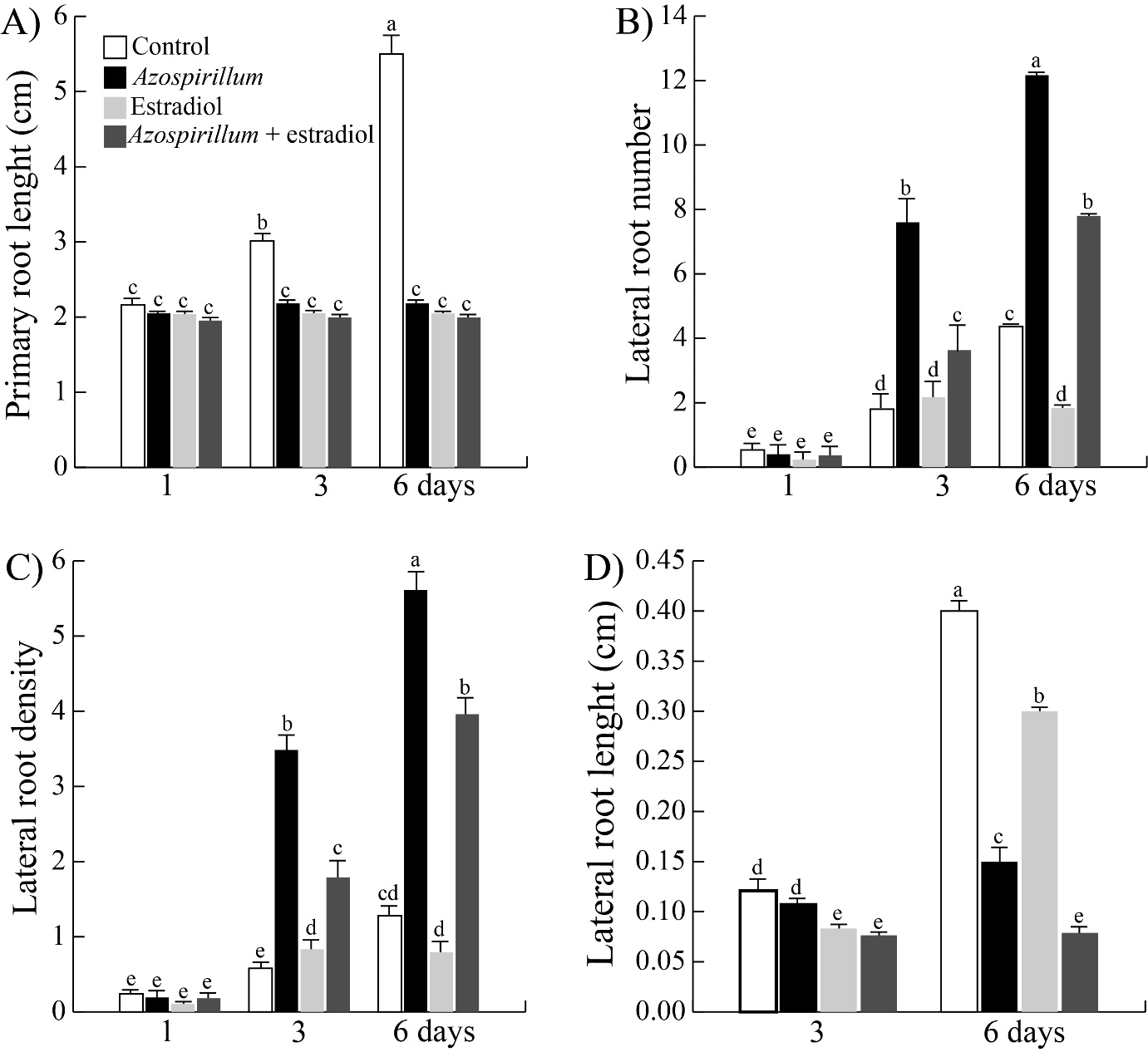
Figure 4 Effect of Azospirillum on the root architecture of tor-es1 seedlings. The estradiol-inducible tor mutant (tor-es1) seedlings were transferred to control media and supplemented with 2.5x10⁵ CFU/mL of Azospirillum, added both media with estradiol (10 µM) and incubated by 1, 3 and 6 days. A) Primary root length. B) Lateral roots number. C) Lateral roots density. D) Lateral roots length. Values represent the mean of thirty seedlings +/- standard error. The letters indicate the statistically significant differences between the parameters of the Tukey test (P≤0.05). The experiments were repeated in triplicate with similar results.
Participation of TOR on the polar transport of auxins during the Arabidopsis-Azospirillum interaction
Next, the effect of PAT inhibition on the estradiol-inducible tor mutant (tor-es1) exposed to Azospirillum was evaluated, for which tor-es1 seedlings were transferred to culture media supplemented with 2.5X105 CFU/mL of A. brasilense and 1 µM NPA in the presence and absence of 10 µM estradiol. The material was incubated for one, three and six days and the root architecture parameters were evaluated. In Figure 5A, it can be seen that in all the treatments the root length was reduced in the times tested, however in presence of NPA, there was a certain resistance to that effect. The seedlings in the presence of NPA plus the bacteria decreased the lateral roots number by 50% on the third and sixth day. However, in seedlings exposed to NPA plus A. brasilense and in the absence of TOR (presence of estradiol), a reduction by 26 and 56% of root branching was observed on the third and sixth days of interaction, respectively (Figure 5B). Meanwhile, NPA together with Azospirillum inhibited lateral roots length on the third day by 40% and it increased by 30% at 6 days of exposition. While in the absence of TOR and inoculated with A. brasilense plus NPA, lateral roots length showed a 42% decrease and an increase of 78% on the third ant six days of interaction respectively (Figure 5D).
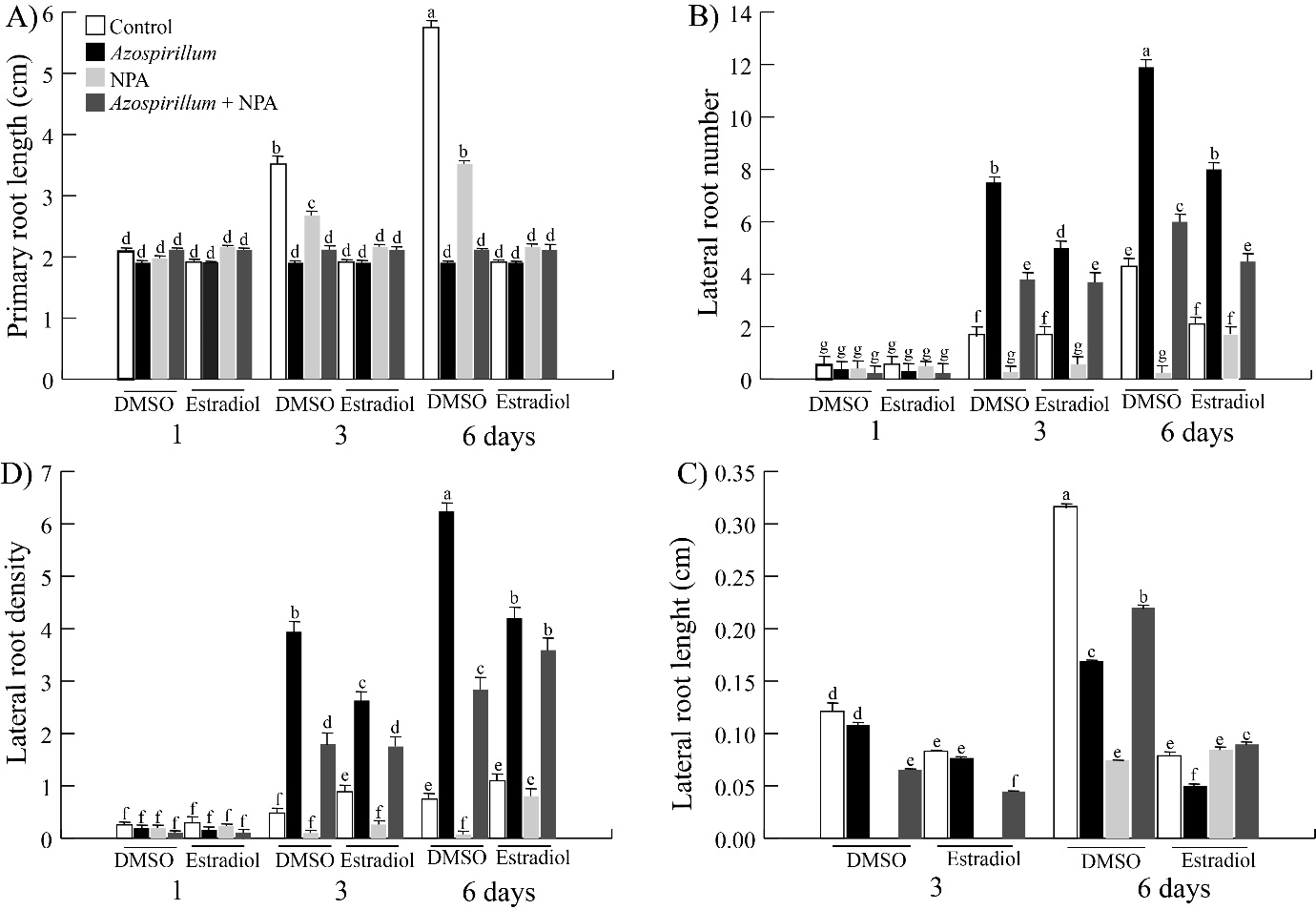
Figure 5 Effect of PAT inhibition on the root architecture of tor-es1 seedlings exposed to Azospirillum. The estradiol-inducible tor mutant (tor-es1) seedlings were transferred to control media and supplemented with 2.5x10⁵ CFU/mL of Azospirillum, added both media with estradiol (10 µM) and NPA (1 µM) and incubated by 1, 3 and 6 days. A) Primary root length. B) Lateral roots number. C) Lateral roots density. D) Lateral roots length. Values represent the mean of thirty seedlings +/- standard error. The letters indicate the statistically significant differences between the parameters of the Tukey test (P≤0.05). The experiments were repeated in triplicate with similar results.
Involvement of TOR on lateral root growth of Arabidopsis exposed to Azospirillum
Primary root growth is due to both root meristem cell division and cell elongation.32 To analyzed which process would be affected in the decrease on lateral roots growth in presence of Azospirillum and when TOR is inhibiting (Figure 1A), cell division was first analyzed. For this, the line CycB1::uidA was used, which expresses this gene in the G2/M phase of cell cycle.26 It was observed that from the third day of exposure, CycB::uidA marker was expressed in the lateral root meristem in a similar way to the control, while in the seedlings exposed to A. brasilense and AZD-8055, a decrease in this marker was observed and a disappearance at sixth day (Figure 6A). On the other hand, to assess the effect of the bacteria on cell elongation, the behavior of Exp7::uidA on seedlings, which specifically marks the differentiation zone of primary root was analyzed.27 In Figure 6B, it was observed that from the third day of exposure that Exp7::uidA expression extends towards the root tip and the strength in the elongation zone decreased, while in presence of AZD-8055, that expression disappeared completely after three and six days of interaction with the bacteria. Together these results show that Azospirillum represses lateral roots growth, inhibiting both cell division and elongation, thus promoting cell differentiation at very short exposure times to the bacteria.
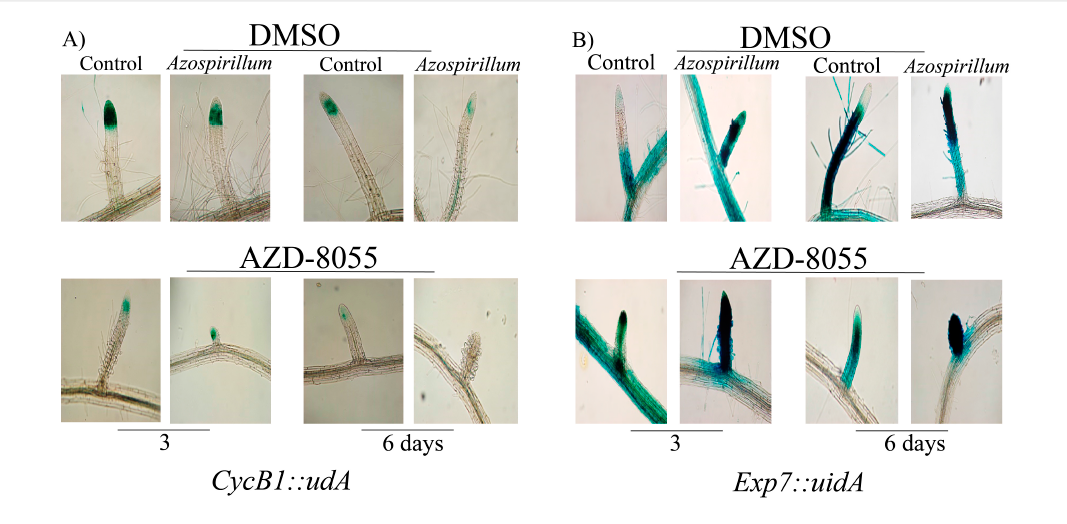
Figure 6 Effect of TOR inactivation on CyB1::uidA and Exp7::uidA expression in Arabidopsis lateral roots. A) Arabidopsis CycB1::uidA and b) Exp7::uidA seedlings were transferred to control medium and supplemented with 2.5x105 CFU/mL of Azospirillum, added both media with AZD-8055 (1000 nM) and incubated by 3 and 6 days. At the end of the incubation time, the seedlings were treated with X-Gluc, followed by a clearing process. The seedlings were mounted on slides and microscopic observations were made at 4X magnification. Representative photographs of the lateral roots from the seedlings in the control medium and inoculated with Azospirillum of at least 25 seedlings from three different trials with similar results are shown.
Azospirillum brasilense has been reported to arrest the primary root length and increase the root branching in A. thaliana seedlings exposed at the rhizobacteria for six or seven days.17–19 In the present work, changes in root architecture induced by Azospirillum were determined at shorter exposure times. The results showed that the rhizobacteria arrested primary root growth throughout all incubation times, while the lateral roots number increased after three and six days of exposure to the bacteria from the first day of interaction (Figures 1A&B). Thus, the effect of the bacteria on the root system occurs at very short exposure times. As this Arabidopsis root phenotype induced by A. brasilense has been attributed to high concentrations of bacterial IAA,16,17 we analyzed auxin levels in Arabidopsis DR5::uidA seedlings exposed to the bacteria. We observed that the level of auxins increased in primary root vascular tissue, in the differentiation zone of lateral roots; this effect intensified by day fourth of exposition, while that the maximum of auxins in primary root meristem disappear from fifth day of interaction with the bacteria and the auxins accumulated in vascular tissue are redistributed to lateral root meristems (Figure 1E). This relocation of the auxin marker (Figure 1E) suggests that PAT could be involved during the Arabidopsis-Azospirillum interaction. To test this hypothesis, we inhibited PAT with NPA and found that the inhibitor blocks the stimulatory effect of the bacterium on the lateral root number (Figure 2B), it suggests that PAT is involved during the Azospirillum-Arabidopsis interaction. These results are similar to those reported by Wang et al.,33 where Arabidopsis seedlings inoculated with Bacillus sp LZR216 and in the presence of NPA, showed a decrease in the root branching compared to the control. Reed et al.,34 when applying NPA to Arabidopsis seedlings, observed a decrease in free IAA in the roots, as well as a reduction in lateral root number. NPA is known to arrest the development of lateral roots by blocking the division of cells in the pericycle of the xylem pole.35 On the other hand, the participation of TOR during the Arabidopsis-Azospirillum interaction was investigated by two strategies, a pharmacological and other genetic. The results of the first approach showed that AZD-8055 together with rhizobacteria on the third and sixth days decreased the lateral root number by 52 and 36% and the lateral root length by 80 and 45%, respectively (Figures 3(B&D)). While the tor-es1 seedlings presented a reduction of 45 and 33% in the root branching and 38 and 50% in the lateral root length after three and six days of exposure, respectively (Figures 4(B&D)). So, both strategies showed that TOR participates in the stimulation of lateral root development of A. thaliana seedlings inoculated with A. brasilense, effect that was also reported by Méndez-Gómez et al.,18 in Arabidopsis seedlings exposed at Azospirillum in presence of AZD-8055. The results before mentioned are in agreement with what was reported by Zhang et al.,36 who evaluated the participation of TOR in the synthesis of chlorophyll in A. thaliana seedlings, using the inhibitors of TOR, AZD-8055, Torin2, and the mutant raptor1b. Raptor1b seedlings showed a greater reduction in chlorophyll concentration compared to the effect caused by the two inhibitors. These results indicate that inhibiting TOR activity or blocking its transcription does not necessarily lead to the same phenotype. The participation of TOR on PAT was analyzed in Arabidopsis tor-es1 seedlings exposed to estradiol, NPA and in the presence of Azospirillum, which showed a reduction by 20% in the lateral root number on the third day of interaction, with respect to the 50% reduction that occurs in the control treatment (without estradiol) (Figure 5B). Furthermore, the lateral root length also decreased only at 3 days of interaction (Figure 5D). In the present study, we observed that TOR modulates the growth of the lateral roots of A. thaliana inoculated with A. brasilense, arresting cell proliferation and elongation (Figure 6), an effect like that observed in the primary root where the bacterium causes a depletion of the root meristem and a decrease in cell elongation (data not shown).
The participation of TOR during plant-pathogen interaction has recently been evaluated, reports indicate that a decrease in TOR signaling induces plant resistance to pathogen attack.23,37 In this study we observed that Azospirillum stimulates lateral root development, which is decreased by altering TOR signaling, these observations coincide with the results reported by Méndez-Gómez et al., 202018 and Méndez-Gómez et al., 2020.19 Nanjareddy et al.,22 which show that during the Phaseolus vulgaris-Rhizobium interaction, the inactivation of TOR in beans alters the development of the infection thread, which results in the decrease in the size of the nodule induced by Rhizobium.
During the Arabidopsis-Azospirillum interaction, TOR modulates the development of lateral roots through the regulation of cell proliferation and elongation.
We acknowledge J. Sheen for providing tor-esl seeds.
The authors state that there is no conflict of interest.
This work was supported by the Coordinción de la Investigación Cientifíca UMSNH. E.C. –F. and J.A. –R were fellows of CONACYT-Mexico.

©2022 :, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.