Journal of
eISSN: 2572-8466


Research Article Volume 11 Issue 2
Faculty of Agricultural Sciences, National University of Catamarca. Argentina
Correspondence: Di-Barbaro MG, Faculty of Agricultural Sciences. National University of Catamarca, Belgrano Avenue and Maestro Quiroga, (4700). Catamarca, Argentina, Tel +5493834565995
Received: March 26, 2024 | Published: April 12, 2024
Citation: Di-Barbaro MG, Andrada HE, Espeche AER, et al. Effect of the application of plant growth-promoting microorganisms on the cultivation of two varieties of chard (beta vulgaris L. subsp. cicla) under greenhouse conditions. J Appl Biotechnol Bioeng. 2024;11(2):30-33. DOI: 10.15406/jabb.2024.11.00356
Agroecological and sustainable vegetable production arises from the need to produce safe food and protect the environment and with the aim of obtaining sustainable and environmentally friendly agriculture but achieving the best productivity results. Therefore, this work aimed to study the effect of the application of the consortium of native microorganisms on the quality and yield of the crop of two varieties of chard (Beta vulgaris L. subsp. cicla) at the time of transplantation. The trial was carried out in the greenhouse of the Faculty of Agricultural Sciences of the National University of Catamarca. Swiss chard of the white and rainbow varieties was sown in germination trays. The seedlings were transplanted in beds and inoculated with native plant growth-promoting microorganisms at the time of transplantation. Fresh weight and number of leaves per plant and time of harvest were recorded. The design was in randomized blocks with 4 replications; data were subjected to analysis of variance and LSD test (p≤0.05). Plants inoculated with the microbial consortium produced significantly higher yields than control plants. The number of cuts and the yield of the Rainbow variety increased significantly progressively with inoculation.
Keywords: Swiss chard Penca blanca, Arco iris chard, Azospirillum brasilense, mycorrhizal fungi
In a world where environmental awareness and the search for more responsible agricultural practices are constantly growing, the use of inoculants from microorganisms selected for their ability to promote plant growth is presented as an innovative and promising solution.
The use of Plant Growth Promoting Microorganisms (MPCV) has been researched for many years, with the genus Azospirillum being one of the most prominent, mainly due to its ability to produce a wide range of active metabolites such as phytohormones and other plant growth regulators, such as indole acetic acid, cytokinins, gibberellins and siderophores,1,2 which positively influence healthy plant growth and development.3
Among the MPCVs, there are fungi that establish mycorrhizal associations with the roots of most land plants. These mycorrhizal fungi receive carbon compounds from the plant and in return promote plant growth by supplying nutrients from the soil, especially the few mobile ones such as phosphorus and water.4–9 In addition, they confer greater tolerance to pathogen attack and drought.10–11 Mycorrhizae have an advantage over the non-mycorrhizal root because the outer mycelium extends farther than the root hairs, which, from a nutritional point of view, the benefit is the greater growth of the plants due to an increase in phosphorus uptake when this element is limiting, when phosphorus is not limiting the benefit can be null or reduced, according to the degree of mycorrhizal dependence of the plant. In addition, it directly or indirectly influences the absorption of other minerals (N, K, Ca, Mg, Fe, Mn).3,12
Swiss chard (Beta vulgaris L. subsp. cicla) is a biannual vegetable belonging to the Amaranthaceae family, it is a traditional crop that is grown under a conventional system of field production, and highly appreciated by consumers for its antioxidant and anti-cancer properties. However, it is a crop that has been little researched locally, so it is of interest to check the response of the plant to growing conditions. The aim of this study was to study the effect of the application of the consortium of native plant growth promoting microorganisms (MPCV) on the quality and yield of the crop of two varieties of Swiss chard (Beta vulgaris L. subsp. cicla) at the time of transplantation.
The trial was carried out in the greenhouse of the Faculty of Agrarian Sciences of the National University of Catamarca located in the city of San Fernando del Valle de Catamarca (Argentina), in this experiment we worked with two varieties of chard (Beta vulgaris L. subsp. cicla) Penca Blanca and Arco Iris, a traditional variety (Penca blanca) and a new variety little known in the region (Arco iris). In greenhouse conditions and under a production system with an agroecological approach. It was cultivated together with other companion species, such as aromatics, aphid and ant repellents, pollinators, etc., such as basil (Ocimum basilicum), parsley (Petroselinum crispum), tufted flower (Tagetes patula), garden phlox (Phlox sp.). In addition, the conservation and rational use of water resources was promoted, no agrochemicals were used, to achieve a healthy vegetable, a safe food, and the protection of the environment.
Sowing was carried out in germination trays and the seedlings were transplanted into beds with substrate composed of soil, compost, and perlite. Inoculation with native MPCVs was performed at the time of transplantation. Fresh weight and number of leaves per plant and by time of harvest were recorded. The design was in randomized blocks, with 2 treatments, inoculated: with application of microbial consortium of MPCV at the time of transplantation, and control treatment without inoculation; and with 4 replications.
The microbial consortium was composed of native microorganisms: the endorhizospheric bacterium of the genus Azospirillum and mycorrhizal fungi. Inoculation was carried out with the native Pi 8 strain of A. brasilense, isolated from the endorhizosphere of pepper (Capsicum annum var. Trompa de elefante) grown in the Province of Catamarca, whose identification was carried out biochemically and molecularly.1,13,14 The concentration of A. brasilense used for inoculations was 2.5x107 azospyrils. mL-1 quantified in the Neubauer chamber.15 The inoculum of mycorrhizal fungi native to the province of Catamarca was made up of rootlets of Avena sativa L. and Cenchrus ciliaris L. colonized by them. The percentage of mycorrhizal colonization of the roots used as inoculum was 85%, estimated using the method of line intersections and microscopic observation of roots by Sieverding, et al.16,17
Leaf harvesting was carried out at 60, 65, 70, 80, 90, 100, 120, 130 and 145 days after planting, and based on the total fresh mass per plant, the yield of each treatment was estimated.
Data were subjected to analysis of variance and LSD test (p≤0.05) with the statistical program Infostat.18
The results obtained in the two varieties of Swiss chard tested, the highest yields, expressed in fresh weight and number of leaves, were achieved with the inoculation of the microbial consortium of A. brasilense and mycorrhizal fungi, registering highly significant differences, with a p-value of 0.0001 for the number of leaves and a p-value of 0.0008 for the fresh weight of Penca blanca chard leaves and a p-value of 0.0002 for the amount of leaves and 0.0021 for fresh weight of chard leaves of the Arco iris variety (Table 1).
|
Cultivation |
Treatment |
Average sheet weight (g) |
Average number of leaves (g) |
|
White Chard Penca Blanca |
Witness |
39,72 + 26,54 A |
4,55 + 2,10 A |
|
|
Inoculated |
52,68 + 39,05 B |
5,82 + 3,29 B |
|
Arco iris Swiss Chard |
Witness |
69,59 + 75,70 A |
5,85 + 3,31 A |
|
|
Inoculated |
104,92 + 127,49 B |
7,59 + 4,90 B |
Table 1 Comparison of fresh weight and number of leaves per plant of two varieties of Swiss chard (Beta vulgaris L. subsp. cicla) according to treatments
Uncommon letters in the same variable denote significant differences according to LSD test (Minimum Significant Difference) for P<0.05.
In the two varieties of Swiss chard evaluated, the application of the microbial consortium produced results that exceeded the control treatment, results consistent with those obtained by Ardisana et al.19 who studied the effect on the growth of chard (Beta vulgaris L. subsp. cicla) plants of two biostimulants made with vermicompost leachates (Figure 1).

Figure 1 Cultivation of two varieties of Swiss chard (Beta vulgaris L. subsp. cicla) Penca blanca and Arco iris, in a greenhouse with an agroecological approach.
In the Penca blanca variety, 7 harvests were carried out, where an average production per plant during the entire crop cycle was 5,968.8 g in the control plants and 8,639.1 g in those inoculated with the microbial consortium (Table 2), while in the Arco iris variety, 6 harvests were carried out and an average yield per plant of 12,039.5 g in the control treatment plants and 16,997.5 g in the control plants inoculated chard plants (Figure 2, Table 3).
|
Days after planting |
Variable |
Treatment |
|
|
|
|
Witness |
Inoculated |
|
60 |
Fresh Weight (g) |
48,92 + 35,14 A |
57,84 + 34,41 A |
|
Number of Leaves |
2,87 + 1,01 A |
3,80 + 1,99 A |
|
|
65 |
Fresh Weight (g) |
38,80 + 22,10 A |
28,83 + 15,07 A |
|
Number of Leaves |
3,86 + 2,03 A |
3,55 + 1,47 A |
|
|
70 |
Fresh Weight (g) |
28,90 + 13,42 A |
25,27 + 11,79 A |
|
Number of Leaves |
5,31 + 2,02 A |
5,27 + 2,18 A |
|
|
80 |
Fresh Weight (g) |
54,46 + 30,59 A |
91,56 + 42,26 B |
|
Number of Leaves |
5,48 + 2,19 A |
9,42 + 3,20 B |
|
|
90 |
Fresh Weight (g) |
41,48 + 28,45 A |
32,92 + 18,12 A |
|
Number of Leaves |
4,67 + 1,63 A |
3,83 + 1,40 A |
|
|
100 |
Fresh Weight (g) |
24,72 + 8,87 A |
22,94 + 14,37 A |
|
Number of Leaves |
5,41 + 2,18 A |
4,95 + 3,29 A |
|
|
120 |
Fresh Weight (g) |
31,76 + 19,49 A |
71,18 + 32,51 B |
|
Number of Leaves |
3,81 + 1,83 A |
6,38 + 2,64 B |
|
Table 2 Comparison of the agronomic parameters of Swiss chard (Beta vulgaris L. subsp. cicla) Penca blanca variety (fresh weight and number of leaves) produced in the FCA-UNCa greenhouse
Uncommon letters in the same variable denote significant differences according to LSD test (Minimum Significant Difference) for P<0.05.
|
Days after planting |
Variable |
Treatment |
|
|
|
|
Witness |
Inoculated |
|
60 |
Fresh Weight (g) |
56,00 + 21,07 A |
84,06 + 46,25 B |
|
Number of Leaves |
3,50 + 1,25 A |
5,72 + 3,29 B |
|
|
65 |
Fresh Weight (g) |
23,92 + 19,14 A |
32,94 + 12,74 B |
|
Number of Leaves |
4,23 + 2,77 A |
5,25 + 2,18 A |
|
|
100 |
Fresh Weight (g) |
126,45 + 79,15 A |
249,96 + 205,55 B |
|
Number of Leaves |
8,28 + 2,55 A |
13,08 + 5,44 B |
|
|
120 |
Fresh Weight (g) |
123,34 + 111,17 A |
162,62 + 135,08 B |
|
Number of Leaves |
8,06 + 3,36 A |
11,02 + 5,44 B |
|
|
130 |
Fresh Weight (g) |
50,17 + 23,39 A |
61,07 + 34,36 B |
|
Number of Leaves |
3,18 + 1,47 A |
5,11 + 2,41 B |
|
|
145 |
Fresh Weight (g) |
31,07 + 21,73 A |
48,18 + 42,68 B |
|
Number of Leaves |
5,11 + 2,38 A |
5,22 + 2,07 A |
|
Table 3 Comparison of the agronomic parameters of chard (Beta vulgaris L. subsp. cicla) variety Arco iris (fresh weight and number of leaves) produced in the FCA-UNCa greenhouse
Uncommon letters in the same variable denote significant differences according to LSD test (Minimum Significant Difference) for P<0.05.
The Arco iris Swiss chard variety was more productive than the traditionally cultivated Penca blanca (Figure 3, 4). The total biomass accumulation of Penca blanca chard coincides with the results obtained by Barrientos Llanos et al.,15 when growing Swiss chard in greenhouses, where the maximum total biomass accumulation value was 114.9 grams, corresponding to the Fordhook giant variety, which generates quite a few leaves, but its quality is essentially that of generating thick and succulent stems.
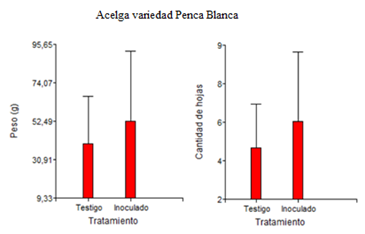
Figure 3 Fresh weight and number of leaves per chard (Beta vulgaris L. subsp. cicla) plant of the Penca blanca variety.

Figure 4 Fresh weight and number of leaves per chard (Beta vulgaris L. subsp. cicla) plant of the Arco iris variety.
These results are consistent with those reported by Campos Martínez et al.21 that evaluated the effect of mountain microorganisms on the production of Swiss chard in the Popayán plateau, a work that evidences the positive effects on the soil and chard plants, thanks to the action of microorganisms, which help to carry out the mineralization of organic matter faster.
As this trial was carried out on a fertile substrate and favorable results were obtained by the inoculation of the microbial consortium composed of Azospirillum brasilense, as it is a bacterium that fixes atmospheric nitrogen and mycorrhizal fungi, which allows us to infer that this biofertilizer will have a greater impact on soils of lower fertility.22,23
Therefore, it is concluded that inoculation with the microbial consortium composed of the bacterium Azospirillum brasilense and native mycorrhizal fungi positively influences the cultivation of the varieties of Swiss chard (Beta vulgaris L. subsp. cicla) Penca blanca and Arco iris, improving yield, health and safety, fundamental characteristics to achieve the success of the crop. This shows the potential of the bacterial strain and mycorrhizal fungi tested as a biofertilizer for the agroecological production of chard crops.
In addition, the present work provides information on a non-traditional variety in the region (Arco iris), while highlighting that the deepening of knowledge on the subject would contribute to a better understanding of the processes involved in the production of Swiss chard (Beta vulgaris L. subsp. cicla) in greenhouses with agroecological management.
None.
Authors declare that there is no conflict of interest.

©2024 Di-Barbaro, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.