Journal of
eISSN: 2572-8466


Research Article Volume 9 Issue 5
Department of Genetics, University of Buenos Aires, Argentina
Correspondence: Alicia L Basso, Agronomy Faculty, Department of Genetics, University of Buenos Aires, Argentina
Received: October 15, 2022 | Published: October 26, 2022
Citation: ESchenone C, Basso AL. Double minute chromosomes in Anastrepha fraterculus (Wiedemann) (DIPTERA: Tephritidae): a model for cancer studies. J Appl Biotechnol Bioeng. 2022;9(5):182-188. DOI: 10.15406/jabb.2022.09.00308
Anastrepha fraterculus (Wied.) is known as the South American Fruit Fly. In order to develop efficient control strategies, we need deep knowledge on its biology along with periodical studies on population dynamics. Citogenetic studies on natural populations of this pest fly made it possible to reveal the presence of double minute chromosomes (DMs) in several natural populations of this insect. Cytogenetic studies performed in our insect laboratory, allowed the genetic analysis through a genealogical methodology. The establishment of families made it possible the genetic studies allowing the rigorous identification, characterization and confirmation of new chromosomal variants, frequently missinterpreted when the materials analyzed only come from nature. Double minute chromosomes -considered a type of chromosomal rearrangement- are extra chromosomal gene copies. This study sought to answer: What role do DMs chromosomes play in laboratory populations of the pest derived from natural populations? How are they transmitted from one generation to another? To answer our questions, we analyzed for 25 generations, two laboratory populations derived from Tucuman (T) and Buenos Aires (BA -tester) guava,. Data were recovered from cytological analysis of ganglia preparations revealed with H33258. The natural population from BA didn’t carry DMs. Results showed DMs in flies of both laboratory populations. DMs were transmitted from parents to progenies through 25 generations and their transmission was randomized in number. DMs are the cytological expression of resistance mechanisms used by the pest as a response to environmental stress. DMs in BA strain marked the change to the laboratory environment. A. fraterculus is a model insect for the study of cancer.
Keywords: cytological markers, environmental contamination, stress, chromosomal rearrangements, genic amplification
All living organisms need the cell cycle to increase in size and/or replace dead cells.
The cell cycle consists of three distinct phases: interphase, mitosis, and cytokinesis. Before a eukaryotic cell can begin mitosis and divide, it must replicate its DNA, synthesize histones and other proteins associated with the DNA of the chromosomes, produce an adequate supply of organelles for the two daughter cells, and assemble the structures necessary for them to intersect. carry out mitosis and cytokinesis. These preparatory processes occur during the interphase of the cell cycle, in which, in turn, three stages are distinguished: the Gl, S and G2 phases.1
During the S phase (of synthesis) the chromosomal material is duplicating. Between cell division and the S phase there are two G phases (G= gap= interval). The first of these (G1) is a period of general growth and duplication of cytoplasmic organelles. During the second (G2), chromosome condensation begins and the assembly of structures directly associated with mitosis and cytokinesis begins. After the G2 phase, mitosis occurs where the duplicated chromosomes are distributed between the two daughter nuclei.
The breeding of A. fraterculus under laboratory conditions allows genetic studies to be carried out by families, rigorously identify genetic variants (chromosomal, biochemical, molecular), confirm and characterize new mutants, frequently misinterpreted when studies are only carried out on materials that come from nature. Thus it is possible to understand the meaning of variability.
The most common karyotype is composed of 5 pairs of telocentric autosomes, an acrocentric X chromosome, and a small submetacentric Y chromosome, such that 2n = 2x = 10 + XX/X.2 The heteromorphic pair is generally associated with the pair of XX or XY sex chromosomes which remain separate during mitotic metaphase.
During the S phase (of synthesis) the chromosomal material is duplicating. Between cell division and the S phase there are two G phases (G= gap= interval). The first of these (G1) is a period of general growth and duplication of cytoplasmic organelles. During the second (G2), chromosome condensation begins and the assembly of structures directly associated with mitosis and cytokinesis begins. After the G2 phase, mitosis occurs where the duplicated chromosomes are distributed between the two daughter nuclei.
Finally, in cytokinesis, the cytoplasm divides, separating the mother cell into two identical daughter cells. When no more cells are required, they enter a state called G0, in which they leave the cell cycle and enter a period of latency.1
Cells continually monitor their external environment as well as their internal physiological state and functions. In the absence of necessary nutrients or growth factors, animal cells can exit the cell cycle and enter a resting state called G0. Following growth stimulation, cells re-enter the cell cycle.3
Cells have mechanisms that respond to stress symptoms, including DNA damage, oxygen depletion, inadequate amounts of nucleoside triphosphates, and (in the case of animals) loss of intercellular adhesion. Within the cell, several key events in the cell cycle are monitored. When defects are identified, cell cycle progression stops at a checkpoint allowing time for correction and repair. The checkpoint serves to maintain the correct order of the phases of the cell cycle.3
There are three key checkpoints in the cell cycle: DNA damage checkpoint, checkpoint at centrosome duplication and checkpoint in the mitotic spindle (Figure 1). Failure at any checkpoint in the cell cycle results in genetic instability leading to different types of chromosomal mutations.
The malfunction of the mitotic spindle can lead to aneuploidy, while an error in centrosome duplication can lead to polyploidy. Failures at the DNA damage checkpoints (Figure 1) can result in chromosomal aberrations of various types, including translocations, deletions, and amplifications of genes or chromosome regions. Amplified genes can be found as tandem repeats within a chromosome or extrachromosomal circles that lack a centromere as well as telomeres.3
Gene amplification
Amplification is defined as a molecular process that results in an increase in the number of copies of a discrete chromosomal region of DNA.4,5 In increase in the number of copies of a gene. There may also be an increase in the RNA and protein made from that gene. Gene amplification is common in cancer cells, and some amplified genes may cause cancer cells to grow or become resistant to anticancer drugs. Genes may also be amplified in the laboratory for research purposes.
These types of mutations have been observed in a large number of human tumors including breast, lung, ovarian, colon cancer, leukemias, and neuroblastoma.6–14 Clinically, the amplification has prognostic and diagnostic utility and is a mechanism of acquired drug resistance.16,17
Double minute chromosomes (DMs)
DMs are acentric circular fragments without telomere that replicate autonomously. Having no centromere, segregate randomly at mitosis and may be lost during cell division unless they confer a proliferative advantage to cells, such as when they carry amplified drug resistance genes.4,7
DMs chromosomes were observed and described for the first time in A. fraterculus by Basso18sup>in natural populations of Argentina. DMs and homogeneously stained regions (HRS) are two cytogenetic manifestations of gene amplification.19 They can be located as extrachromosomal elements (DMs) or within the chromosomal arm, giving rise to homogeneously stained regions (HSR).4 The presence of DMs in cells of cancer patients is an indicator that the administered drug has ceased to be effective and should be replaced by another.10
This work aims to answer the following questions:
What role do DMs chromosomes play in laboratory populations of the fruit fly derived from natural populations? How are they transmitted from one generation to another?
Our working hypothesis is that DMs are the cytological expression of resistance mechanisms used by the pest in response to stress situations.
Materials
We utilized a wild type laboratory strain named 294 and a laboratory colony named EEAOC derived from natural populations of A. fraterculus of different geographic origins.
Strain 294 was established from 1 male and 1 female flies emerged from guava fruits originated in Buenos Aires (Latitude: 34° 39’ 25.29” S; Longitud: 58° 40’ 31.43” O). EEAOC colony was established from a group of specimens recovered from guavas collected in Tucumán (Latitud: 26° 48’ 29.83” S; Longitud: 65° 13’ 03.32” O). EEAOC is the Experimental Agroindustrial Station Obispo Colombres in Tucumán Province.
Both stocks were mantained in the Cytogenetics’ Laboratory of fruit flies at Agronomy School, University of Buenos Aires, under controlled conditions: temperature (24ºC), humidity (80%) and fotoperíod (16 hs light and 8 hs darkness) accordingly to the A. fraterculus breeding technique for genetic studies developed and described by Dr. Fanny Manso.2
Methods
Cytology-Third instar larvae were isolated and the cerebral ganglia was dissected following the protocol previously described by Basso. This organ has a high rate of cell division (mitosis). Cytological preparations were obtained following the protocol by Willhoeft & Franz. The slides were placed in coplin with a 3:1 solution of Methanol: Acetic Acid and kept in a freezer at -20ºC until used. Hoechst staining was performed following the technique by Gatti et al.20 modified by Basso.
Statistical analysis
For statistical analysis the Student InfoStat Program version 2017 was used. A box plot (Box Plot) was made to know how the observed data are distributed, a T-Student test to check if there are differences between population means, at 1%; and a Chi-square test of homogeneity to verify if the proportions of individuals carrying DMs from EEAOC and 294 are equal to those expected, at 1%.
During two years and a half we analyzed 22 generations of flies.
Detection of double minute chromosomes (DMs) and homogeneously stained regions (HSR).
DMs are not brillant to Hoescht 33258 and were observed in metaphase nuclei (Figures 1A, 1B) as well as in mitotic interphase (Figures 2A & 2B).
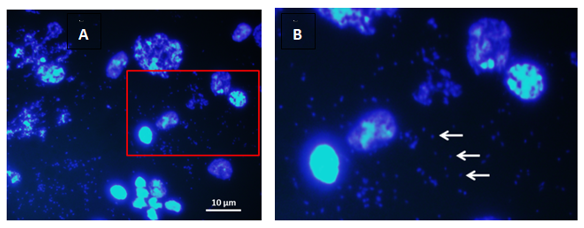
Figure 2 Doble minutes (DMs) in interphase célls: A) Panoramic view of nuclei brilliantly stained with Hoechst 33258. Note the small opaque spheres around the nuclei that are in pairs. B) field extension. White arrows indicate doble minute chromosomes. 1000x.
The cytological study of 90 preparations from strain 294 and 115 preparations from the EEAOC colony show that two thirds of them are dividing nuclei providing reliable data, and a part of these correspond to individuals carrying double minute chromosomes (DMs) (Table 1).
H-banding |
Ner of preparations |
Ner of preparations with reliable data |
Ner of preparations with DMs |
Strain 294 |
90 |
63 |
58 |
EEAOC Colony |
115 |
74 |
50 |
Table 1 Total number of preparations per stock over number of preparations showing reliable data
Presence of DMs in populations from different geographical origin
We studied the changes in the composition within each geographic population concerning presence/absence of DMs/specimen thoughout generations. The first two generations here analyzed, evidenced that 50% of individuals from 294 strain carried DMs while only 30% individuals from colony EEAOC did so (Table 2).
|
Buenos Aires |
Tucuman |
Insects with DMs |
50% |
30% |
Table 2 Percentage of individuals carrying DMs (294 n=10; EEAOC n=10) at the beginning of this study
During the following 16 generations the number of individuals carrying DMs gradually increased within both 294 strain and EEAOC Colony. From the 18th generation on, DMs were observed in 100% individuals from strain 294 and in 73,43% individuals from EEAOC colony (Table 3).
|
Buenos Aires |
Tucuman |
Insects with DMs |
100% |
73,43% |
Table 3 Percentage of individuals with DMs after 22 generations (294 n=53; EEAOC n=64)
During the nineteenth generation, DMs were fixed in the strain 294.
Data obtained experimentally are clear evidence for strong differences between both populations from different geographic origins.
Statistical analysis
For the statistical analysis, we used the variable total number of DMs per individual. This is due to the impossibility of defining which core each DM belongs to. In the annex are the data produced by the InfoStat program.
Summary measures of the total of each sample
The media, standar deviation, variance, coefficient of variation, minimum and maximum value of both materials for the variable “total number of DMs per individual” (Total DMs/individual) (Table 4).
Population |
Variable |
N |
Media |
SD |
Var(n-1) |
CV |
Mín |
Máx |
A-294 strain |
DMs/ind |
58 |
489,00 |
587,34 |
344968,77 |
120,11 |
21 |
3162 |
|
|
|
|
|
|
|
|
|
B- EEAOC |
DMs/ind |
50 |
132,06 |
249,49 |
62244,83 |
188,92 |
5 |
1367 |
Table 4 Summary measures for 294 and EEAOC (InfoStat, 2017)For a better visualization of the data, a box-plot graph was made (Graph 1).
In Graph 1 we can see that the distribution for the total number of DMs/specimen in both populations is not simetric (data are far from a normal distribution) and the mean of strain 294 is greater than EEAOC.
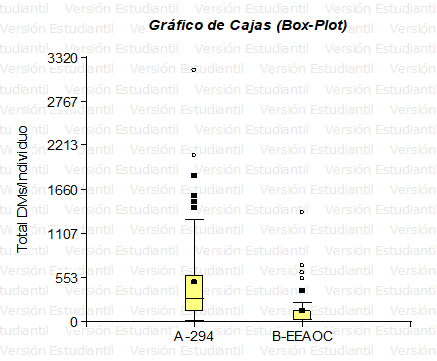
Graph 1 Box Plot for the variable “Total number of DMs/specimen” (Info Stat). The boxes show the interquartile ranges, the black dots (on yellow) indicate the mean, and the points outside the bar the outliers (294 n=58; EEAOC n=50).
Relative and absolute frequency of DMs
The frequencies for the variable "total DMs/Individual" in both populations are shown in Table 5.
Population Variable Class IL SL CM AF RF |
A-294 Total DMs/ind. 1 [ 21,00 649,20 ) 335,10 46 0,79 |
A-294 Total DMs/ind. 2 [ 649,20 1277,40 ) 963,30 5 0,09 |
A-294 Total DMs/ind. 3 [ 1277,40 1905,60 ) 1591,50 5 0,09 |
A-294 Total DMs/ind. 4 [ 1905,60 2533,80 ) 2219,70 1 0,02 |
A-294 Total DMs/ind. 5 [ 2533,80 3162,00 ] 2847,90 1 0,02 |
Population Variable Class IL SL CM AF RF |
B-EEAOC Total DMs/ind. 1 [ 5,00 277,40 ) 141,20 44 0,88 |
B-EEAOC Total DMs/ind. 2 [ 277,40 549,80 ) 413,60 2 0,04 |
B-EEAOC Total DMs/ind. 3 [ 549,80 822,20 ) 686,00 3 0,06 |
B-EEAOC Total DMs/ind. 4 [ 822,20 1094,60 ) 958,40 0 0,00 |
B-EEAOC Total DMs/ind. 5 [ 1094,60 1367,00 ] 1230,80 1 0,02 |
Table 5 Absolute frequency and relative frequency of total DMs/individual (InfoStat, 2017) IL, inferior limit; SL, superior limit; CM, class mark; AF, absolute frecuency; RF, relative frequency
We can observe that for line 294 the highest proportion of individuals is located in class 1 (21 – 649.20) with a FR=0.79; and for EEAOC the highest proportion of individuals is also located in class 1 (5– 277.4) with a FR=0.88. Note that line 294 has a higher number of DMs per individual than EEAOC, a fact that is reflected in the class range (LI–LS).
Population mean analysis – T-Student test
The T-Student test for the number of DMs per individual allowed us to demonstrate that the population mean of strain 294 is greater than the mean of the EEAOC colony, at 1% (Figure 3).

Figure 3 A) Female metaphase X1X1 with homogeneously stained regions( HSR); B) Male metaphase X1Y1 with HSR. C) Female X1X1 with HSR.
D – Genetic events related to DMs
In the analyzed individuals, the following phenomena were detected: bridging anaphases, mosaics of nuclei with/without DMs and micronuclei. Here is a summary of the different types of genomic instability found.
Anaphase bridges were observed (Figures 4A&4B) which are initial events in the break-bridge-fusion (BFB) cycles. In the analyzed individuals, the following phenomena were detected: bridging anaphases, mosaics of nuclei with/without DMs and micronuclei. Here is a summary of the different types of genomic instability found.

Figure 4 Comparison between nuclei with and without DMs in individuals from different geographic origin. A) Individual from the EEAOC colony (Tucumán). B) Individual from strain 294 (Buenos Aires) with the cloud of DMs in the yellow circle. 1000x.
Mosaic of cores with and without DMs (Figure 5), show three central interphasic nuclei with DMs and the three adyacent nuclei lack DMs.
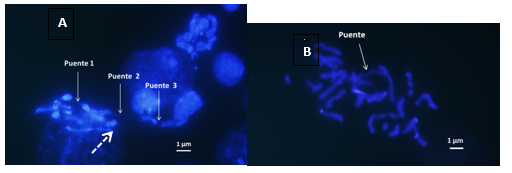
Figure 5 A-Anaphase Bridges 1 and 2 connected by an impercible thin fiber of chromatin; Bridge 3 in anaphase of lagging chromosomes. Dotted arrow indicates HSR. B- The arrow indicate uninterrupted chromatin bridge that will later break and give rise to DMs 1000x.
Micronúclei (MN) were observed in cytological preparations from strain 294 and from colony EEAOC (Figure 5).
Natural populations and laboratory populations
Laboratory materials allow genetic studies to be carried out by families, rigorously identify, confirm and characterize new variants, frequently misinterpreted when the analyzed materials only come from nature.
Our results were possible thanks to this methodology: we managed to study the transmission of tiny double chromosomes from parents to offspring for 22 generations. This evidenced the evolution of the frequencies of individuals carrying DMs over successive generations in the laboratory, highlighting the environment effect (Table 6).
Environment |
||
Locality |
Nature (Basso 1994-2003) |
Laboratory (Present work) |
Buenos Aires 294 |
0% |
100% |
Tucumán EEAOC |
25% |
73.43% |
Table 6 Percentaje of flies with DMs from nature and in Laboratory stocks
Previously, and as a fundamental background, double minute chromosomes had been observed in natural populations of the insect.18 In those studies, the presence of DMs in natural populations of Buenos Aires was not detected.18 Double minute chromosomes were described for the first time in natural populations of an invertebrate during the 90`s, in samples of larvae derived from guava trees in Tucumán, and a physiological adaptive role was proposed.21
In strain 294 derived from Buenos Aires germplasm, the DMs were fixed. This is due to high level of inbreeding within the colony (Figure 6A). In the EEAOC colony derived from Tucumán, the individuals carrying DMs increased their frequency to 73.43% but to a lesser extent compared to strain 294. This is due to the genetic variability that is maintained within the colony (Figure 6B). The increase in the number of individuals with DMs throughout the generations has a genetic component due to high inbreeding, and an environmental one, generated by the change of environment when going from nature to the laboratory breeding chamber.
When the size of the population is small, matings classified by kinship occur that lead to inbreeding and the consequent fixation of alleles (homozygous loci). Due to standarization of the rearing conditions in the laboratory, the mechanism of DMs production evolved in the same direction in both stocks. However, the mean number of DMs per individual was higher in strain 294. These differences between materials are statistically significant at 1% (Table 4 & Graph 1).
Mutations are the raw material for the evolution of species. Its importance becomes apparent in particular during the adaptation of a population to a new environment, either as a consequence of important environmental changes in the place where it lives or because a new geographical area is colonized. In these cases, the selective pressure increases extraordinarily and favors the survival of those individuals that carry the most favorable adaptive mutations.22
Cytogenetic studies of strain 294 and EEAOC colony derived from natural populations and established in the laboratory are clear evidence of the adaptive response to stress generated by laboratory conditions.
DMs and cytological events related to their origin
During this work, events related to tiny double chromosomes, such as anaphase bridges (Figures 4A&4B) and micronuclei (Figure 5) were found. How are these events related? One of the hypotheses that explains how genes are amplified is the breakage-fusion-bridge cycle model (Breakage-Fusion-Bridge = BFB) originally proposed by Barbara McClintock in 1941 (Figure 7).
Organophosphates are carcinogenic compounds. Sometimes at the end of mitosis, they facilitate the production of chromosomal fragments (DMs and HSRs) generated by the breaking of the anaphasic bridges separate from the rest of the genetic material and form structures called micronuclei (MN) (Figures 4 & 5). Micronuclei are defined as extranuclear bodies containing damaged chromosome fragments and/or entire chromosomes that were not incorporated into the nucleus after cell division23 and are formed from a lagging chromosome.
It is relevant to underline that the model “Breakage fusion-bridge” was proposed by Barbara Mc Clintock in 1941. Cancer occurs when cells have changed their genotypic program and involute: they will become a hybrid between a prokaryotic and an eukaryotic cell, something like an archaea (Figure 8).24–43
CRISPR/Cas9 is more famous as one outstanding scientific breakthrough in genome editing. The neoplastic cell acquires resistance to treatment, it acquires characteristics of a high rate of mutation, of response to environmental modifications; it is as if eukaryotic cells regressed and returned to their origins where they were sensitive to the environment and could mutate and then they could have CRISPR systems inside. So this theory says that: a- in cancer the neoplastic cells are secreting exosomes containing DNA; b- that this DNA contains gene editing systems comparable to what would be the CRISPR of bacteria or archaea that would make the neoplastic cells be like a hybrid and could alter their environment, change, mutate and generate mutations in other cells. c- If they see that the exosomes of neoplastic cells are inducing changes and that those changes are being specific in neoplastic or normal cells, then they will identify if that gene is being edited in the genome of the cells.
Using specific Hoechst 33258 staining we analyzed and documented 72 larvae mitosis from strain 294, and 98 from EEAOC during 2 years (16 laboratory generations). Our results showed mosaic insects carrying nuclei with and without DMs, which present anaphases with bridge and fragment and nuclei with incomplete cytokinesis. In 294 and EEAOC we respectively observed and documented: 1- presence of DMs in 100% and 70% of specimens; 2- the mean number of DMs/cell was 9 and 8 and the Rank was 1 to 154 and 1 to 37 respectively.
Double minute chromosomes are the cytological expression of adaptive mechanisms.
During 2 years (16 laboratory generations) using specific Hoechst 33258 staining we analyzed and documented 72 larvae mitosis from strain 294, and 98 from EEAOC.
Our results showed mosaic insects carrying nuclei with and without DMs, which present anaphases with bridge and fragment and nuclei with incomplete cytokinesis.
In 294 and EEAOC we respectively observed and documented: 1- presence of DMs in 100% and 70% of specimens; 2- the mean number of DMs/cell was 9 and 8 and the Rank was 1 to 154 and 1 to 37 respectively.
Double minute chromosomes are the cytological expression of adaptive mechanisms. In A. fraterculus DMs originate from melt-bridge-break, the model described by McClintock in maize in the 1940s..
Transposons are genetic elements able to move within the genome; they are present in animals as well as in plants and,thanks to the Human Proyect Genome, we know in humans more than half of our DNA are transposable elements (although in our case, the majority are inactive) .
The occurrence and development of cancer is a highly complex process with multi-gene and multi-path interactions. Many scientists have been attempting to decipher the mechanisms of cancer occurrence, development, and metastasis and clearly, CRISPR has accelerated research efforts. Presently, CRISPR technology is used to investigate the genetic mechanisms in almost all areas of cancer, from prevention to prognosis and treatment, which greatly promotes transition to the clinic.12
None.
The authors declared no have conflict interest for the study.
None.

©2022 ESchenone, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.