Journal of
eISSN: 2572-8466


Research Article Volume 9 Issue 4
1Chair of Agricultural Microbiology, Biology Department, Argentina
2Phytopathology Chair, Plant Health Department, Argentina
Correspondence: Gabriela Di Barbaro, Chair of Agricultural Microbiology, Biology Department, Faculty of Agricultural Sciences, Research and Postgraduate Secretariat, National University of Catamarca. Avda. Maestro Quiroga 64. (4700). San Fernando del Valle de Catamarca, Argentina
Received: June 29, 2022 | Published: July 14, 2022
Citation: Barbaro GD, Basso VG. Arbuscular vesicular mycorrhizae in olive tree (Olea europaea L.). J Appl Biotechnol Bioeng. 2022;9(4):98-99. DOI: 10.15406/jabb.2022.09.00293
The objective of the work was to determine the general and sanitary condition of olive seedlings (Olea europaea L.). For which the presence of the main microbial group that colonizes the roots of the seedlings of this crop was investigated. To determine and assess fungal root infection, colonization by native fungal organisms in roots of olive trees produced in a nursery in the Central Valley of the province of Catamarca (Argentina) was studied. The studies were carried out by microscopic observation of stained fungal structures within the root, using clarification and staining methodologies. Typical structures of endomycorrhizae such as hyphae, arbuscules and vesicles (MVA), and dark septate endophytic fungi (ESO) with septate, melanized hyphae and numerous microsclerotia were observed. This first condition is desirable in all plants due to its ability to promote plant growth of mycorrhizal-forming fungi.
Keywords: health, endomycorrhizae, MVA, ESO
The national production of olive trees (Olea europaea L.) takes place mainly in Catamarca, which forms the producing nucleus of Argentina together with the provinces of La Rioja, San Juan and Mendoza. In all fruit plantations, including olive cultivation, it is essential to start with excellent quality seedlings to obtain the maximum economic benefit. Therefore, it is necessary to study the condition of the seedling before undertaking the plantation. The objective of this work was to determine the general and health status of olive seedlings. For which the presence of the main microbial group that colonizes the roots of the seedlings of this crop was investigated.
Root treatment: The roots of 5 (five) olive seedlings (Olea europaea L.) were extracted and washed with running water, then the thinnest were selected, which were clarified and stained following the methodology of Phillips & Hayman1 for the detection of mycorrhizal structures (Figure 1). Staining was performed with Gueguén's triple dye solution, which allows fungi to simultaneously stain proteins in blue, starch in violet, fats in red2 and glycogen in mahogany.3
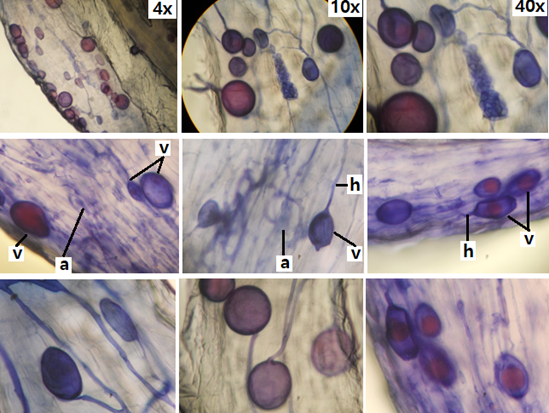
Figure 1 Micrographs of mycorrhizal structures in olive roots (Olea europaea L.a, arbuscules; h, hyphae; v, vesicles
Microscopic examination: The roots were mounted between slides and coverslips, for each specimen microscopic preparations were made. The roots were observed using an optical microscope with standard objectives of 10, 40 and 100x.
Typical structures of vesicular-arbuscular mycorrhizae (VAM) were observed: hyphae, arbuscules and vesicles, in the roots of all the olive seedlings analyzed. The hyphae are continuous thin and thick, some of them with rosary lipids inside with intracellular and intercellular growth. Numerous vesicles of diverse morphology (spherical, oval, tapered and irregular) were observed (Figures 1), so it is inferred that the rootlets of this plant are colonized by various species or genera of native mycorrhizal fungi.
The vesicles were shown with light blue (saccule) and red (single or multiple globules) colorations (Figure 1). These structures are related to carbon storage in the form of lipids and fatty acids, which is why vesicles are defined as reserve organs of the fungal symbiont.4
In addition, septate melanized hyphae with numerous microsclerotia of the type of dark septate endophytic fungi (ESO) were observed5 (Figure 2). The roots of all the analyzed olive seedlings were simultaneously colonized by both endophytes, MVA and ESO, due to the presence of vesicles, VA mycorrhizal arbuscules and ESO microsclerotia.
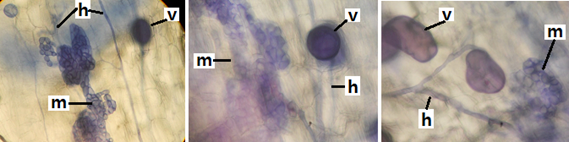
Figure 2 Microsclerotia (m) of the type dark septate endophytic fungi (ESO) in olive rootlets (Olea europaea L). Vesicles (v), hyphae (H). (A: 40x).
Figure 2 shows the presence of vesicles and microsclerotia in an olive root, indicating double colonization by mycorrhizae and dark septate endophytes. This was also observed in various groups of plants, including ferns, mono and dicots that are simultaneously colonized by MVA and ESO.6–9
The mycorrhizal association in olive seedlings (Olea europaea L.) with the co-occurrence of vesicular arbuscular mycorrhizae and dark septate endophytes native to the Central Valley of the Province of Catamarca is described. Under natural conditions, the roots of olive trees are mycorrhized and it is considered that the fungus and the plant have evolved together to be more efficient, and the presence of this beneficial association in olive seedlings is a desirable condition to undertake the implantation and health of the olive tree crop.
None.
The authors state that there is no conflict of interest.
None.

©2022 Barbaro, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.