Journal of
eISSN: 2572-8466


Review Article Volume 11 Issue 2
1Department of Chemistry and Industrial Chemistry (DCCI), University of Genoa, Italy
2Department of Bioengineering, University of California (UCLA), USA
Correspondence: Bill Tawil, Department of Bioengineering, UCLA School of Engineering, 420 Westwood Plaza, Room 5121, Engineering V. P.O. Box: 951600, Los Angeles, CA 90095-1600, USA, Tel +(310)794-5956
Received: April 29, 2024 | Published: May 3, 2024
Citation: Andrea O, Bill T. A review on biopolymer-based bioinks for 3D bioprinting. J Appl Biotechnol Bioeng. 2024;11(2):43-52. DOI: 10.15406/jabb.2024.11.00359
3D bioprinting is a technology currently evolving for extensive applications within tissue engineering and regenerative medicine. The increasing demand for organ transplants and the limited supply of suitable donors have sparked significant interest in 3D bioprinting as a viable solution to organ scarcity. 3D bioprinting involves the use of a specialized biomaterial known as bioink. This medium is made up of cells embedded within a hydrogel or another type of matrix, enabling the creation of complex living tissues. Bioinks are crucial in building functional scaffolds or constructs by precisely depositing them in a pre-arranged pattern to form three-dimensional structures layer by layer. The demand for bioinks in tissue engineering, regenerative medicine, and pharmaceutical drug development is rising, leading to a steady increase in the bioink market over the next decade. In 2022, the market size is valued at 154.97 million USD, and it is projected to reach 571 million USD globally by 2029. This increasing market demand spurs the creation of different biotech companies specializing in the creation of bioinks for 3D bioprinting. This paper explores various bioink materials, including the essential properties of a bioink crucial for 3D bioprinting, as well as current market trends, commercially available bioink products, and companies considered to be key players in the bioink industry, demonstrating its potential growth and the ongoing need for innovation in bioink development to meet the expanding demands in biomedical applications. Further, this paper also discusses the manufacturing process of bioinks, which includes the three main stages of the bioprinting process, as well as the most commonly used bioprinting techniques. The review underscores the importance of advancing bioink technology to enhance the efficacy and utility of 3D bioprinted tissues and organs, enabling the creation of transplanted tissues tailored uniquely for individual patients.
Keywords: bioink, biopolymers, 3D bioprinting, organ printing, tissue engineering, regenerative medicine
3D, three dimensional; ECM, extracellular matrix; AM, additive manufacturing; CT, computed tomography; MRI, magnetic resonance imaging; CAD, computer-aided design; CAM, computer-aided manufacturing; HDF, human dermal fibroblast; HUVEC, human umbilical vein endothelial cell; MSC, mesenchymal stem cells; HSCs, hepatic stellate cells; PTEC, proximal tubular epithelial cells; hiPSCs, human-induced pluripotent stems cells; EBB, extrusion-based bioprinting; LAB, laser-assisted bioprinting; STL, stereolithography
In recent years, three dimensional (3D) bioprinting has been gaining traction as a revolutionary technology in bioengineering and regenerative medicine.1 Recent statistics show that many individuals died while on the waiting list for a life-saving organ, mainly due to the increasing demand for organ donors which vastly exceeds the current supply.2 In this regard, 3D bioprinting presents an opportunity to bridge the current issue of organ scarcity as it has produced notable breakthroughs in biomaterials and cell synthesis to create composite living tissues.3
3D bioprinting utilizes a specialized biomaterial called bioink, which is a bio-printable medium consisting of cells suspended in a hydrogel or other matrix material, to produce intricate living tissues.3,4 These bioinks are applied in the fabrication of functional scaffolds or constructs through a printing process that strategically places the bioinks in a predefined manner to construct 3D structures layer-by-layer.5 Bioinks have many distinct medical applications, ranging from tissue engineering,6 drug delivery,7 cancer therapy,8 and organ printing.9 This paper will be focusing on the different kinds of bioinks commonly used in 3D bioprinting.
Types of bioinks
In all 3D bioprinting applications, the primary and most crucial step is the mindful selection of the bioink to be used.10 Bioinks can be broadly classified into two types depending on the source of the material used, natural polymer-based bionks and synthetic polymer-based bioinks.5,10,11
Bioinks made from natural materials provide a biocompatible fabrication process, as they are able to replicate the natural environment of cells and support cell functions, yet they tend to have inferior mechanical strength.5,10 Conversely, bioinks developed synthetically may lack in biocompatibility, but they boast enhanced mechanical characteristics.1 A variety of natural polymers such as collagen, gelatin, fibrin, silk, alginate, chitosan, hyaluronic acid, dextran, cellulose, extracellular matrix, cell aggregates, Matrigel, gellan gum, and konjac gum are utilized in the creation of bioinks.10 In contrast, synthetic polymers commonly employed include polyethylene glycol, polycaprolactone, polyvinylpyrrolidone, poly(L-lactic) acid, and poly(lactic-co-glycolic) acid.10
Currently, bioinks sourced from natural materials are often favored for their exceptional capacity to replicate the biochemical properties of several complex extracellular matrix (ECM) and tissue architecture, presenting minimal cytotoxic effects.1 Moreover, bioinks derived from natural sources have demonstrated an enhanced ability to support cell functions when compared to their synthetic counterparts, making it a superior option in reproducing microenvironments for bioprinted cells.11 Figure 1 below shows a schematic diagram of some natural polymer-based bioinks for 3D bioprinted scaffolds.11
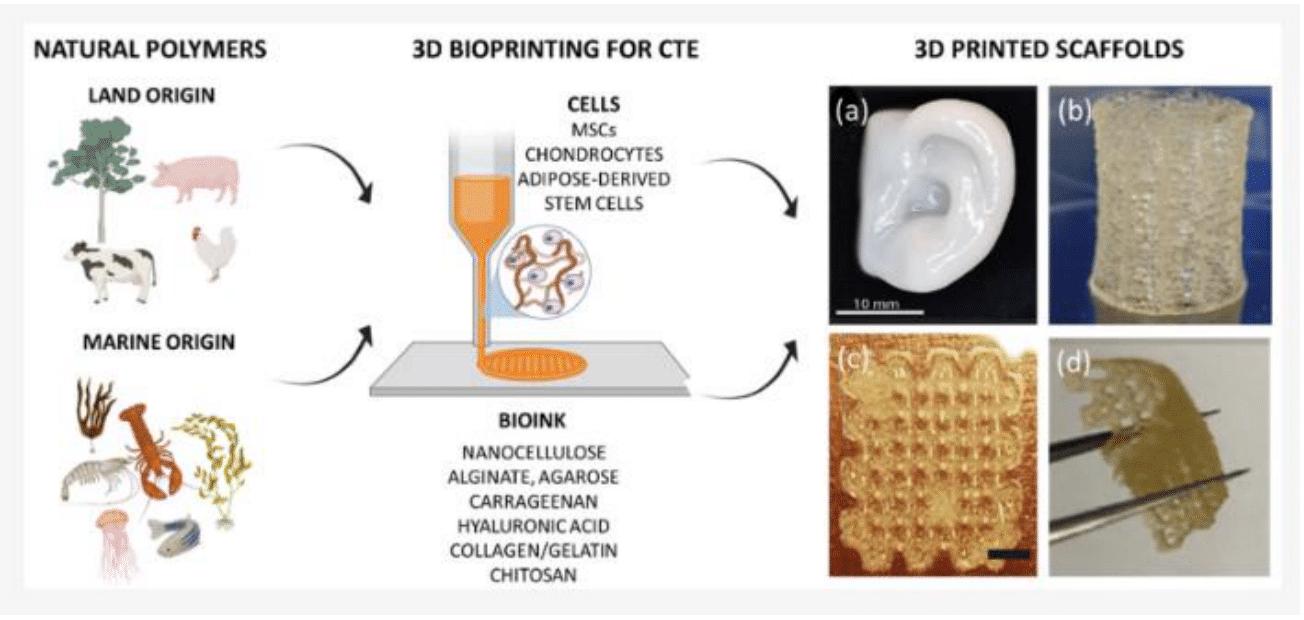
Figure 1 Schematic diagram of some natural polymer-based bioinks for 3D bioprinted scaffolds.11
The image shows the two common sources of natural polymers, different cells used in combination to the bioink material, and examples of resulting 3D printed scaffolds.
Bioink properties essential for 3D bioprinting
A bioink considered to be ideal for 3D bioprinting is defined as a bio-based material that excels in three key areas: printability, biocompatibility, and mechanical properties.12,13
Printability
To ensure printability of bioinks, it is necessary to ensure that the biomaterial has suitable rheological and gelation properties.10,12,13 The rheological properties of bio-ink, like viscosity, are crucial in the printing process, where excessive viscosity can cause gel formation leading to cell death, nozzle blockages, and the creation of self-supporting filamentous structures, while optimal viscosity ensures even cell distribution and facilitates mixing without significantly reducing cell viability.12,14 Moreover, the characteristics of bioink can be altered by the pressure experienced as it is extruded through the nozzle, making it essential for an ideal bioink to behave as a shear-thinning fluid under pressure and to retain its structural integrity post-extrusion by exhibiting a viscoelastic response.5,10,15
Additionally, the solidification of bioink from a gel state after extrusion, known as the gelation process, impacts both cell viability and the resolution of bioprinting.10 Optimizing the gelation time of bioinks is essential not only for supporting self-sustaining structures but also for avoiding uneven cell distribution and preventing nozzle blockages.12
Biocompatibility
Biocompatibility denotes a bioink's ability to safely engage with recipient tissues and physiological systems, ensuring no adverse local or systemic responses arise from interactions with cells, tissues, and the immune system.4 A biocompatible bioink must sustain high cell viability, foster cell growth and proliferation, maintain the healthy, characteristic phenotype of the cell population, and avoid causing cytotoxicity, premature stem cell differentiation, or triggering inflammatory responses in the host.1
Moreover, biomimicry is an important aspect to be considered especially for cell-laden bioinks, as the essential role of any biomaterial ink is to mimic the ECM of living tissue, thereby enabling natural cell adhesion and morphology.1,16 In addition, since cells are an essential element of bioinks, careful selection of cell type and course is crucial to ensure the bioink's cellular function and efficiency.10
Mechanical properties
The mechanical properties of a bioink are characterized by its durability to resist forces that native tissues face during the bioprinting process, while possessing suitable biodegradability properties.12 The components of the bioink must offer post-printing mechanical support and allow for gradual biodegradation to enable tissue remodeling and maturation, without breaking down during the printing process.1 The degradation of the material scaffold leads to the embedded cells secreting proteases and producing ECM proteins that shape the new tissue, and the byproducts should be non-toxic, easily metabolizable, and excretable from the body.10
Market size, products and companies
Due to the increasing use of bionks in tissue engineering, regenerative medicine, and pharmaceutical drug development, the current market for bioinks is forecasted to steadily grow in the next 10 years.17-19 In 2021, it was reported that the global revenue from the bionk market reached USD 115.7 million;17 in 2022, the market size was valued at USD 154.97 million;18 while in 2024, the worldwide bioink market is estimated to be valued at USD 185.6 million.19
These reports collectively highlight the growing market size of bioinks, as shown in Figure 2 below, with its compound annual growth rate (CAGR) estimated to increase by 20.48%, effectively reaching a market size of USD 571 million by the end of 2029.18
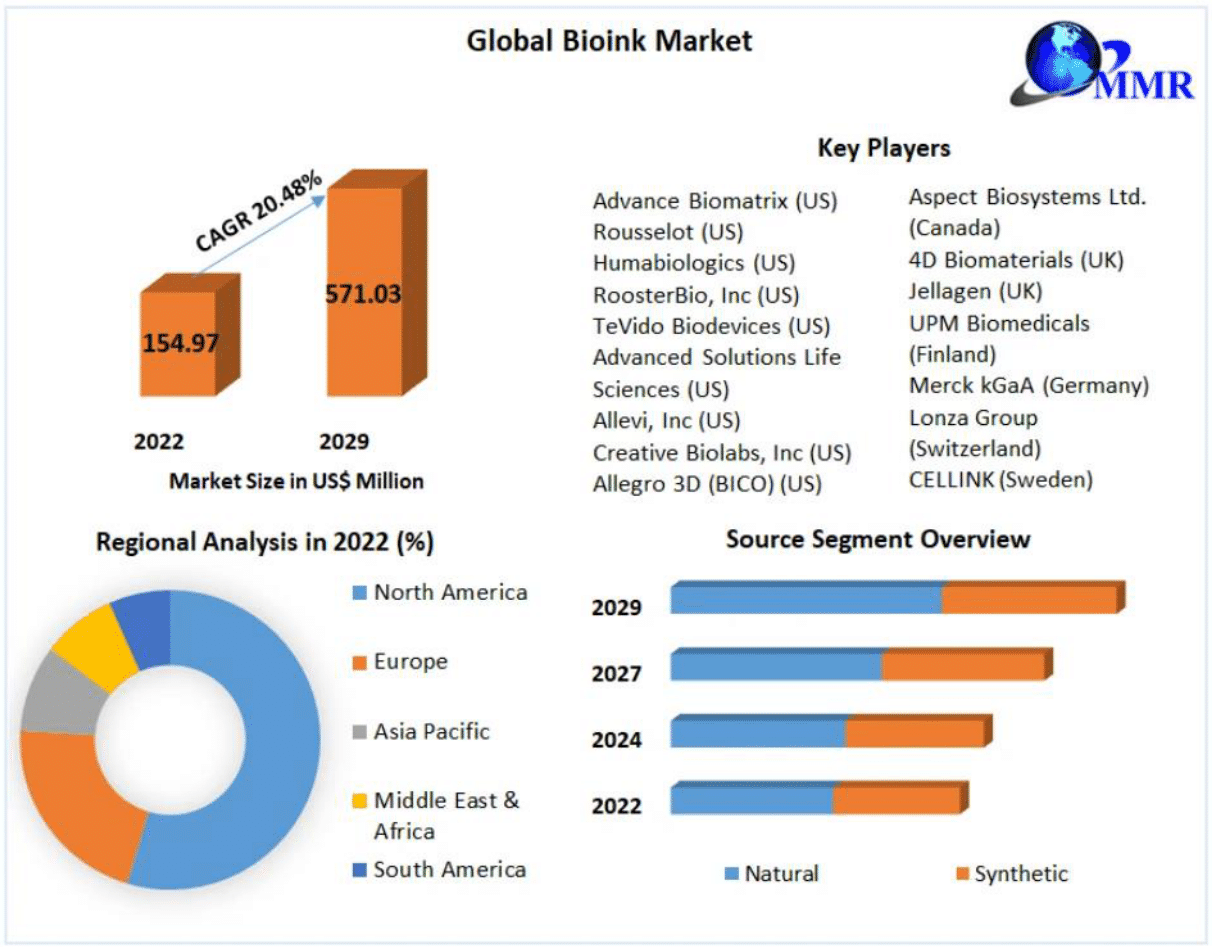
Figure 2 2022 Global bioink market overview.18
The image shows an overview of the 2022 Global Bioink Market, including some company key players, regional analysis, and source segment overview.
Global bioink market size and trends
The rising demand for tissue and organ transplants, on top of the significant developments in 3D bioprinting in recent years, are driving the growth of the global bioink market.17-19 Furthermore, various companies, academic institutions, and government agencies from different parts of the world have been recently investing in bioink research and development, which adds to the driving factors of the bioink market growth.17,18 For instance, in the United States, the government introduced Additive Manufacturing (AM) Forward, an initiative aimed to assist the country’s small-and-medium-sized manufacturers in their 3D printing needs.20
In terms of regional market, the United States dominated the North American bioink market at 95% share, while Germany led the European bioink market at 28.1%.17 The Asia Pacific region is also starting to expand its market presence with a collective revenue share of 23.7% in 2022, with Japan and China leading the region.18 In particular, the Chinese government is actively working towards self-sufficiency in the biopharmaceutical industry, marking it as one of the nation's key objectives, hence it is channeling billions into biotechnology R&D to achieve this goal.17,19
Bioink leading natural sources and products
Bioinks also accounted for 8.9% of the global 3D bioprinting market in 2021.17 Among the most commonly used biopolymer-based bioinks, collagen-based bioinks were reported to have held the largest share from 2021-2022 (20.3% in 2021 and 19.5% in 2022).17,18 Other leading natural bionk sources include alginate, gelatin, hyaluronic acid, fibrinogen, and chitosan (Table 1).1-10
|
Bioink |
Application |
Commercially-available bioink |
References |
|
Alginate-based |
Wound healing, drug delivery, tissue engineering |
PhotoAlginate®-INK CELLINK |
10, 2a |
|
Chitosan-based |
Tissue engineering (bone, cartilage, skin) |
Chitoink by CELLINK, |
12, 2e, 2f |
|
ChiMa Bioink by Adbioink |
|||
|
Collagen-based |
Regenerative medicine, tissue engineering |
Humaderm by Humalogics, |
27, 2g, 2h |
|
rHCollagen by Collplant |
|||
|
DECM-based |
Tissue engineering (skin, heart, intestines) |
Matrigel by Corning Life Sciences |
10, 2i |
|
Fibrin-based |
Scaffold bioprinting, tissue engineering |
TissuePrint by Axolotl Biosciences |
28, 2d, |
|
Gelatin-based |
Tissue engineering (cardiac valves, cartilage) |
X-Pure® by Rousselot, |
5, 2b, 2c |
|
Gelatin Methacrylate by Allevi 3D Systems |
|||
|
Hyaluronic Acid-based |
Tissue engineering (brain, bone, cartilage) |
HAMA Bioink by Adbioink |
10, 2j |
Bioink companies – key players
These bionks are developed and commercialized by a number of biotech companies and start-ups reported to be the key players currently moving the global bioink market, summarized in Table 2 below.21
|
Name of Company |
Year Founded |
Location |
Products |
Reference |
|
BICO Group (formerly CELLINK) |
2016 |
Sweden |
CELLINK: Tissue-specific bioinks, medical grade bioinks, photoinks |
1a |
|
Advanced Biomatrix: 3D hydrogels, ECMs, HyStem/Hyaluronic Acid |
||||
|
CollPlant |
2004 |
Israel |
rhCollagen, Collink 3D bioinks |
1b |
|
Rousselot |
1891 |
USA |
Gelatins, hydrolyzed gelatins, collagen proteins/peptides |
1c |
|
Humabiologics, Inc. |
2018 |
USA |
Human collagen, human gelatin, matrix proteins, human tissue/organ ECM |
1d |
|
Axolotl Biosciences |
2020 |
Canada |
TissuePrint |
1e |
|
Allevi 3D Systems |
2014 |
USA |
Human and animal-derived bioink sources, specialized bionks for liver, heart, bone, cartilage, kidney, etc. |
1f |
Table 2 Bioink keyplayers and their specializations21
Among the aforementioned companies, BICO Group is considered one of the major and most established bionk companies in the world.22 Formerly CELLINK, BICO aims to broaden bioprinting research within academic circles by offering its affordable CELLINK series of bioprinters with an extensive variety of bioink materials, enriched by their acquisition of Advanced BioMatrix in 2021.23,24 In 2021, the company secured intellectual property rights for a cellulose-based bioink, following the patenting of their printing hardware designed to adjust temperature for optimal flow and cell viability.22 As of 2022, BICO Group’s overall revenue amounted to around USD 217 million, of which USD 60.2 million is from their bionks and bioprinting segment.24
Another notable company is CollPlant, which introduced an innovative plant-based technology standing as the sole commercially feasible method for the mass production of recombinant human Type I collagen (rhCollagen), perfectly mirroring the collagen naturally produced by the human body.21,25 In 2022, the company reported a USD 299,000 revenue which was mainly generated by the sales of their bioink products and rhCollagen.25-28
Rousselot, a brand under Darling Ingredients, is widely considered a leading company in collagen-based solutions.1c Their X-Pure® line of bioinks is an ideal material for bioprinting cell-laden scaffolds, as it suitably mimics the ECM.21 Similarly, an Arizona-based startup called Humabiologics has been gaining attention for being the first company to develop and commercialize native human collagen bionks and gelatins.1d
Another noteworthy startup is Axolotl Biosciences, which won the esteemed SpinOff prize from Nature journal as a scientific startup of note on the year it was established.22 Their acclaim is based on TissuePrint, a fibrin-based bioink capable of printing Human Induced Pluripotent Stem Cells (hiPSCs), Neural Progenitor Cells (NPCs), and Mesenchymal Stem Cells (MSCs).22,1e
Lastly, 3D Systems has been starting to mark its presence in the bioink and bioprinting market through its acquisition of the startup Allevi in 2021.23 Recently, the company has also announced the formation of Systemic Bio, a new bioprinting subsidiary which aims to 3D bioprint vascularized organ models using human cells.29
Manufacturing process
Bioinks for commercial purposes are developed through a series of steps aimed at ensuring they are suitable for 3D bioprinting applications, such as tissue engineering, regenerative medicine, and drug testing.6-9 There are three commonly identified phases in the wider 3D bioprinting manufacturing process, which are pre-bioprinting, bioprinting, and post-bioprinting, and the preparation of bioinks will be discussed further within the first step, which is the pre-bioprinting step.3,10,39 Identifying a suitable bio-ink is crucial in 3D bioprinting because it creates a tissue-specific microenvironment essential for supporting cell growth and development.40
Each step in the process, shown in Figure 3, enhances the efficiency of the resulting constructs and has the potential to affect subsequent steps.3
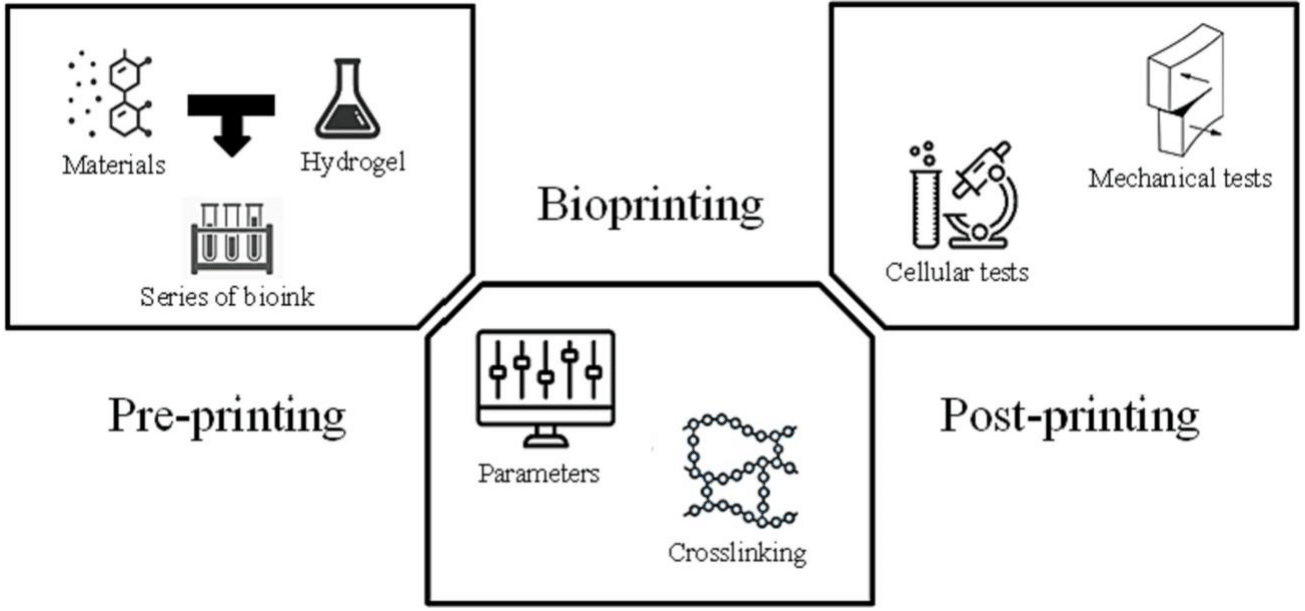
Figure 3 Overview of the general phases of 3D bioprinting.10
The image shows a schematic overview of the three general phase of 3D bioprinting, which includes pre-bioprinting, bioprinting, and post-bioprinting.
Moreover, for the successful fabrication of bioprinted tissues or organ-like structures that promote cell proliferation, creating a comprehensive set of printing instructions and choosing the right bioprinting materials, such as bio-inks and cells, is crucial.3,10,40 Additionally, controlling the bioprinter before beginning the fabrication process and conducting quality control checks after printing are critical steps.40 Figure 4 shows a typical 3D bioprinting manufacturing workflow.40
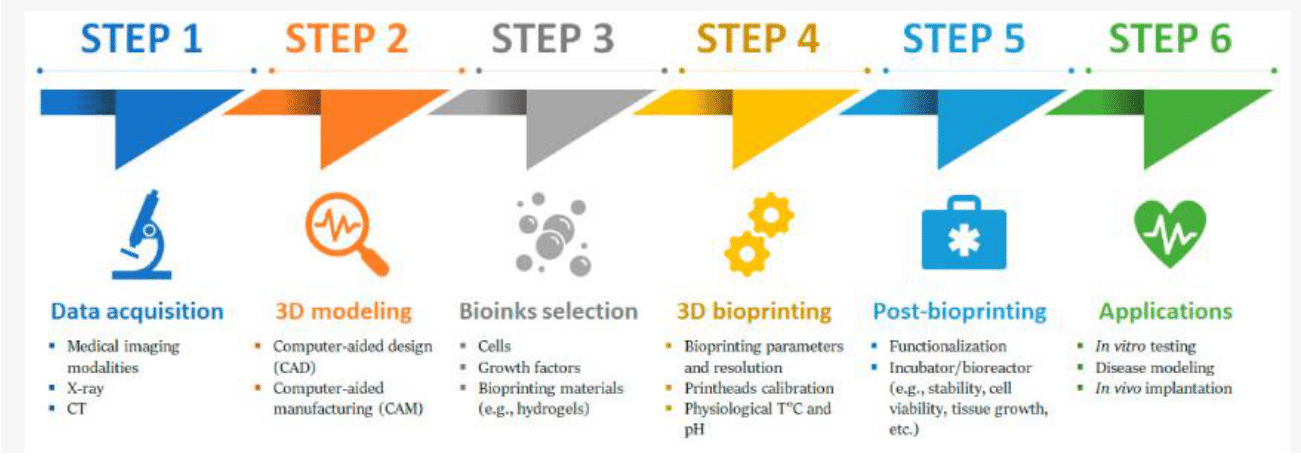
Figure 4 Typical 3D bioprinting manufacturing workflow.40
The image shows a typical manufacturing workflow for 3D bioprinting, which includes steps such as Data Acquisition, 3D modeling, Bioinks selection (under Pre-bioprinting), 3D bioprinting, Post-bioprinting, and Applications.
Pre-bioprinting
The pre-bioprinting stage usually involves acquisition of data through medical imaging modalities, 3D modeling of the desired bioprinted construct, and selection/preparation of cell types and bioink materials.37,40
Data acquisition and 3D modeling
In the first step, a digital file is developed for the bioprinter, incorporating three-dimensional models derived from imaging data to represent tissue or an organ accurately.10,37,40 Imaging data used to create a design for a biological model can be gathered using techniques such as X-ray, computed tomography (CT), or magnetic resonance imaging (MRI), or they can be directly generated using computer-aided design (CAD) software.10,37,40 Afterwards, computer-aided manufacturing (CAM) software is employed to confirm the feasibility of the output, and subsequently, the print file is transformed into a stereolithography (STL), which is a format readable by the printer.40 It is important to note that within various companies, these steps may vary, taking CELLINK as an example which uses its software called DNA Studio that allows its researchers to directly create simple three-dimensional geometries on the printer itself.41,42
Cell type selection
Cells are an essential element of bioink, with the chosen cell type and source being crucial in determining the constraints of the whole printing process.10 In cell-laden bioinks, concentrations can vary between 1 to 10 million cells per milliliter, with a single bioprinting experiment potentially needing up to 50 million cells.43 Given the high number of cells required in bioink formulation, cell lines are frequently utilized due to their affordability and accessibility, yet they commonly fall short in functionality.5 To address this issue, many researchers are shifting towards the use of stem cells, as primary cells are strenuous to culture and often have limited life.10
The ideal bioink incorporates autologous cells, those taken from the patient, to prevent immune rejection.44 Hence, the chosen primary cell type needs to be resilient, capable of withstanding the stresses of printing techniques and the crosslinking process.44 Various cell types have been previously used in 3D bioprinting, and Table 3 below shows a summary of the some of the typically used cells and bioinks in relation to its target tissue model.45–53
|
Tissue Model |
Cells Used |
Bioink Used |
Reference |
|
Muscle |
Myoblasts (C2C12 cell line) |
Nanofibrillated Cellulose (NFC)/alginate-fibrinogen |
|
|
Skin |
Human Dermal Fibroblast (HDF), Human Umbilical Vein Endothelial Cell (HUVEC) |
Silk fibroin and gelatin |
|
|
Bone |
Mesenchymal Stem Cells (MSC) |
Gelatin Methacrylate (GelMA) |
|
|
Liver |
Hepatic Stellate Cells (HSCs) |
Liver ECM |
|
|
Heart |
Human stem cell-derived cardiomyocytes, cardiac fibroblasts |
Collagen Type I |
|
|
Intestine |
Human Intestinal Myofibroblasts |
Silk Fibroin |
|
|
Lung |
Mesenchymal Stem Cells (MSC) |
Sodium Alginate and Gelatin |
|
|
Kidney |
Proximal Tubular Epithelial Cells (PTEC) |
Gelatin/Alginate |
|
|
Brain |
Human-induced Pluripotent Stems Cells (hiPSCs) |
Matrigel and Alginate |
Table 3 Summary of commonly used cells and bioinks in various tissue models45–53
Bioink selection
3D bioprinting depends significantly on bioinks, biomaterials, and cells for generating 3D cultures, with these bioinks necessitating functionality and biocompatibility to replicate the properties of living tissues.10 In selecting the appropriate bioink for 3D bioprinting, it is essential to be familiar with their general functions, which are typically categorized into four: structural, functional, sacrificial, and support.10
Structural bioinks are the biomaterials responsible for giving 3D printed structures their form and stability, and can also serve as durable sacrificial bioinks.54,55 These bioinks uphold the construct's mechanical integrity, facilitate cell adhesion, proliferation, and differentiation, and emulate the ECM as cells multiply.37 Functional bioinks provide biochemical, mechanical, or electrical cues that influence cellular behavior following the printing of a structure.37,54,55 This often includes biomaterials which are nano-functionalized and loaded with various growth-factors.54
Sacrificial bioinks, also known as 'fugitive bioinks,' are typically printed apart from the bioink to create open spaces by being removed post-printing.55 Drawing inspiration from tissue engineering techniques that employ materials like salt particles, beads, and sugars to form pores in biomaterial scaffolds, these sacrificial inks are frequently utilized to engineer hollow structures within a print, akin to vasculature networks.54,56 Support bio-inks are typically biomaterials that support cell population during the printing process and serve as a matrix similar to the extracellular environment while the cells grow.54,55 They are commonly applied to enhance bioprinted constructs due to the inherent softness and low structural integrity of hydrogels, and are particularly beneficial in the creation of connective or hard tissues like cartilage, bone, or muscle (Table 4).43
|
Bioink Type |
Function |
Bioink Material |
Application |
|
Structural |
- upholds mechanical integrity of construct, facilitate cell adhesion, proliferation, differentiation37,54,55 |
Bone regeneration,57 tissue engineering and localized drug delivery,58 skin model and wound healing59 |
|
|
Functional |
- provide biochemical crosslinking support following printing37,54,55 |
Periodontal regeneration,60 skin bioprinting and wound healing,61 corneal stromal model62 |
|
|
- used for gelation of other bioinks10 |
|||
|
Sacrificial |
- bionks that are removed after printing55 |
Vascularized tissues,61 Heart scaffold and liver tissue formation61 |
|
|
- provide temporary support and typically used in engineering vascular networks10,54,56 |
|||
|
Support |
- usually used to enhance mechanical properties of constructs10,43 |
Gelatin-methacryloyl64 |
Artificial ovaries64 |
Structural, functional, and support-type bioinks are usually prepared in the same manner, in which the chosen bioink material is dissolved in a liquid medium (e.g. distilled water, ammonium hydroxide solution, lithium bromide solution, RPMI 1640 medium, deionized water, McCoy’s 5A medium) following manufacturer’s instructions.46-64 Afterwards, if the bioink is multicomponent, an additional material is mixed into the solution to obtain the target concentration.59,61 Generally, multicomponent bio-inks are favored over single biomaterial-based bioinks because they can maintain maximal cell viability by mitigating the varying toxic effects each biomaterial may have on different cells.54 Then, bioink solutions undergo dialysis for a number of days and at an identified temperature, which varies according to the physiological temperature and requirements of the target printed structure.40,46,48 Finally, the solution is freeze dried or lyophilized to removed excess water while preserving the biological components within the bioink.46,48 Figure 5 shows a schematic example of a typical bioink preparation process.46
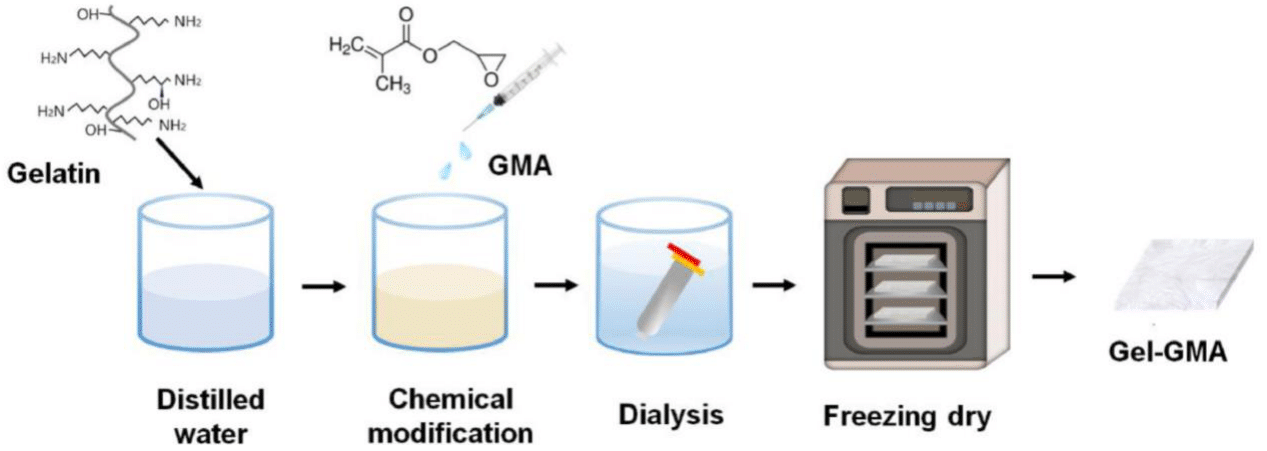
Figure 5 Schematic example of a typical bioink preparation process.46
The image shows an example of a typical bioink preparation process for GelGMA. Gelatin was dissolved in distilled water and allowed to react with GMA overnight. Subsequently, to eliminate salts, the bioink solutions underwent dialysis against distilled water, followed by freeze drying.
Unlike the three previously mentioned bioink types, sacrificial bioinks are different in that they act as temporary support and are removed post-printing.37 Sacrificial templates are integrated into low-viscosity bioinks that contain effector cells and are then allowed to cure properly.65 Once adequate crosslinking is achieved, these sacrificial materials are removed through methods such as solvent dissolution, temperature adjustment, or pH modification.65 In addition, sacrificial bioinks function similarly to support baths and thus necessitate Bingham viscosity, meaning they remain solid under low-stress conditions but transform into a viscous fluid when subjected to high stress.10 This property facilitates material displacement during bioprinting and provides critical mechanical support to delicate bioinks.10
Once an appropriate bioink type and material is selected, the chosen cell type previously isolated is then added to the solution, in varying concentrations aligning with the requirements of the chosen printed construct.40 Before bioprinting, a live-cell imaging system is utilized to confirm the presence of a sufficient number of cells for the successful creation of a tissue model.40
Bioprinting
In the bioprinting step, the prepared bioink is loaded into the bioprinter and then bioprinted onto a scaffold to form a three-dimensional structure, which is based on the 2D design outlined in the software.10,37 The bioprinting process starts with feeding the previously collected data from the CT/MRI scans of the preferred model to the bioprinter.10,40 The bioprinter will then process the data and deposit the biomaterial onto the receiving platform, which is accomplished through the multidirectional movement of the print head that allows for the creation of the desired depth and thickness.10 Figure 6 shows a schematic overview of the different techniques used for 3D bioprinting.3
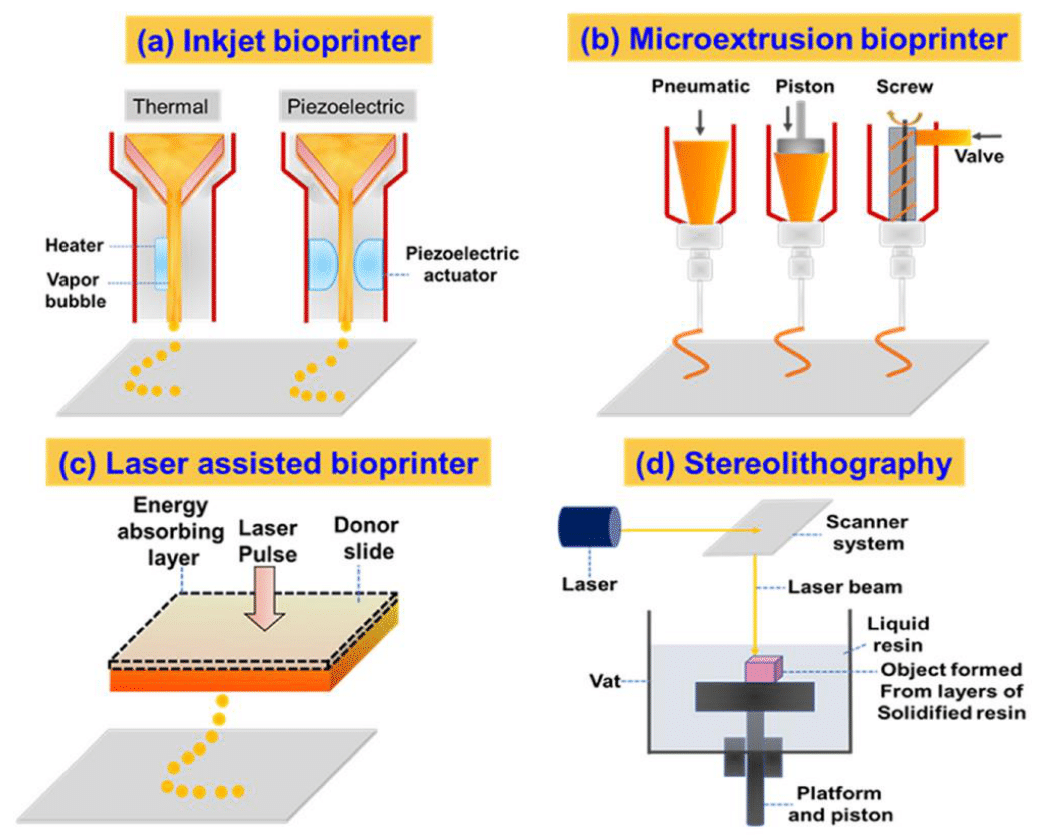
Figure 6 Overview of the different techniques used for 3D bioprinting.3
The image shows the four most commonly used techniques in 3D bioprinting, which includes Inkjet bioprinting, Extrusion-based bioprinting, Laser-assisted bioprinting, and Stereolithography.
In 3D bioprinting, there are four commonly used bioprinting techniques: inkjet bioprinting, extrusion-based bioprinting, laser-assisted bioprinting, and stereolithography.3 Table 5 below shows a summary of common bioink materials and their corresponding bioprinting method as identified from recent literature.
|
Bioink Material |
Bioprinting Method |
Reference |
|
Alginate |
Extrusion-based bioprinting, laser-based bioprinting, inkjet bioprinting |
|
|
Chitosan |
Extrusion-based bioprinting, laser-based bioprinting |
|
|
Collagen |
Extrusion-based bioprinting, droplet-based bioprinting |
|
|
Fibrin |
Extrusion-based bioprinting, droplet-based bioprinting |
|
|
Gelatin |
Extrusion-based bioprinting, STL, inkjet bioprinting |
|
|
Hyaluronic Acid |
Extrusion-based bioprinting, STL |
Inkjet bioprinting
Inkjet bioprinting utilizes a microelectro-mechanical systems (MEMS) process, employing thermal bubble or piezoelectric-driven jetting of microdrops, which is known for its low cost, high precision, and rapid speed.65 Inkjet bioprinting encompasses both continuous and drop-on-demand methods.40 While continuous inkjet printing faces challenges with conductive bio-inks and high contamination risks due to ink recirculation, drop-on-demand bioprinting is crucial for its ability to accurately place well-distributed, cell-laden bio-ink droplets.3,40 These droplets are expelled through the nozzle by a pressure pulse in the microfluidic chamber, controlled by thermal or piezo actuators, making it highly suitable for bioprinting applications.40 For inkjet bioprinting, the size of ink droplets varies between 10 to 150 µm, necessitating bioink formulations with very low viscosities (<10 mPa·s) and demanding rapid, thorough layer-by-layer crosslinking to create 3D structures, consequently restricting the range of materials suitable for this technique.65 Inkjet bioprinters account for 10% of the global market share of commercial bioprinters, which has been employed to fabricate a variety of 3D tissues and biological structures to date, such as cartilage replicas, engineered neural, brain, renal, and smooth muscle tissues, and other complex heterogeneous tissue constructs.65
Extrusion-based bioprinting (EBB)
Presently, extrusion-based bioprinting is the most widely used bioprinting method, preferred for its simplicity, versatility in material choices (such as polymer melts, hydrogels, dECM, nano-clays, etc.), and cost-effectiveness.3,66 Unlike inkjet bioprinting, in EBB, bioink is dispensed as a continuous filament instead of individual droplets, thus facilitating the bioprinting of highly viscous formulations and allows for the incorporation of higher cell densities.65 Typically, an extrusion-based bioprinting setup includes a printing head, a computer-controlled three-axis positioning system, and a printing stage.5,40 This system maneuvers the printing head across the X, Y, and Z axes relative to the printing stage, enabling the deposition of bioink from a loaded syringe onto the stage.5 EBB offers benefits such as minimal material constraints, the ability to incorporate high cell densities, and excellent cell viability after printing, making it adaptable for crafting complex compositional and structural constructs.35 However, its moderate printing speed and resolution present challenges to scaling up this bioprinting method.35,66 Extrusion-based bioprinters represent 57% of the commercial bioprinters of the global 3D bioprinting market, and is currently mainly used within various research institutions for tissue and organ research.10,40
Laser-assisted bioprinting
Laser-assisted bioprinting (LAB) is an advanced technology that utilizes a laser to precisely deposit hydrogel microdroplets of biomaterials onto a substrate through a laser-assisted transfer method.3 A typical laser-assisted bioprinting setup comprises a pulsed laser beam, a focusing system, a "ribbon," and a receiving substrate opposite the "ribbon."10 The "ribbon" includes a donor transmission support, usually glass, coated with a layer that absorbs laser energy (like titanium or gold), atop which sits a layer of biological material (such as cells within or without hydrogel) in liquid form.10 This bioprinting approach is notable for its outstanding resolution, enabling the fabrication of high-resolution tubular capillary structures with diameters as small as 10 µm and the processing of bioinks with up to 95% cell density or highly viscous support materials, up to 1 Pa s, without the risk of nozzle clogging.65 However, the high cost of equipment necessary for laser-assisted bioprinting and its limited adoption within the scientific community constrain the scalability of this bioprinting method.35 In the global 3D bioprinting market, laser-assisted bioprinters account for 3%, with POIETIS (based in Pessac, France) being the sole company dedicated to manufacturing this type of bioprinter.40
Stereolithography (STL)
Stereolithography (STL) offers a stable and nozzle-free approach for crafting 3D structures from diverse biological and non-biological materials.3 It excels in fabricating complex components with high precision, employing light-sensitive hydrogels that are layered to construct a 3D form.3 During the STL process, also known as vat photopolymerization, a UV laser processing light-emitting device illuminates and solidifies a liquid photopolymer resin—a type of thermosetting plastic—layer by layer to create 3D objects.10 STL-based bioprinting offers significantly greater printing resolution and speed compared to extrusion-based methods, making it well-suited for industrial manufacturing adaptation; however, its reliance on photocrosslinkable biomaterials restricts its broader application possibilities.35
Post-bioprinting
A key phase in the bioprinting workflow involves transforming a biomaterial solution or bioink into a gelled or crosslinked hydrogel.5 This occurs during crosslinking in post-bioprinting, in which physical and chemical stimuli are applied to stabilize the mechanical properties of bioprinted structures, hence resulting in a hydrogel that possesses a structurally stable polymeric network.5,10,37 Although numerous methods exist, the most commonly used crosslinking techniques in bioprinting primarily include ionic, thermal, photo, and enzyme-induced crosslinking (Table 6).5
|
Bioink material |
Crosslinking or gelation methods |
Advantages |
Limitations |
Application |
Reference |
|
Alginate |
Ionic, photo crosslinking |
- cost effective |
- bioinert |
- bone or cartilage |
|
|
- good biocompatibility |
- limited long-term stability |
- cardiovascular |
|||
|
- high cell viability |
- limited mechanical stability |
- liver |
|||
|
- constant viscosity |
- muscle |
||||
|
- nerve |
|||||
|
Chitosan |
Thermal crosslinking |
- good ECM compatibility |
- long gelation rate |
- bone |
|
|
- good cell adhesion, proliferation |
- poor mechanical properties |
- skin |
|||
|
- blood vessels |
|||||
|
- neural |
|||||
|
- cornea |
|||||
|
Collagen |
Thermal, Photo crosslinking |
- good biocompatibility |
- poor mechanical properties |
- spine |
|
|
- low immunogenicity |
- low viscosity |
- vascular |
|||
|
- dental |
|||||
|
- heart tissue |
|||||
|
Fibrin |
Enzymatic crosslinking |
- good biocompatibility |
- fast degradation |
- wound healing |
|
|
- high cell adhesion |
- poor mechanical properties |
- drug delivery |
|||
|
- tissue regeneration |
|||||
|
Gelatin |
Thermal, enzymatic crosslinking |
- biocompatible |
- poor mechanical properties |
- cartilage |
|
|
- biodegradable |
- low viscosity |
- heart tissue |
|||
|
- accelerates gelation rate |
- ligament |
||||
|
- cornea |
|||||
|
Hyaluronic Acid |
Physical and chemical crosslinking?? |
- good biocompatibility |
- poor mechanical properties |
- drug delivery |
|
|
- good shear thinning |
- no direct gelation |
- wound healing |
|||
|
|
|
- visco elastic properties |
|
- odontology |
|
Different bioinks used in studies and clinical trials
Different kinds of bionks have been reportedly undergoing clinical trials for numerous biomedical applications.30-31 For instance, biomaterials based on collagen are currently utilized in clinical trials for a range of biomedical purposes, such as treating muscle atrophy, aiding lymphatic system regeneration, and healing chronic ulcers.30 It was also reported that several dECM bioinks, such as photo-crosslinkable cartilage-derived dECM bioinks, cartilage-derived dECM bioinks, liver-derived dECM bioinks, and skin-derived dECM bioinks, are employed in research laboratories and clinical trials.31 Furthermore, scientists conducted a clinical trial comparing microfracture treatment to the implantation of BST-CarGel, an acellular scaffold made of chitosan polysaccharide, and found that compared with traditional surgical strategies, BST-CarGel applied to debrided cartilage lesions resulted in a more stable and adherent blood clot.32
In addition, various clinical trials on the utilization of different kinds of bioinks for 3D bioprinting have also been conducted in recent years.33–35 One study presented a fast-cross-linking bioink capable of creating spheroids of various diameters in a scalable manner with a commercial drop-on-demand bioprinter, which demonstrates a potential scalable platform for drug screening, enabling the investigation of various treatments across different cancer stages.33 Figure 7 shows the schematic process for the bioprinting of a breast tumour spheroid from fast-cross-linking bionks.33
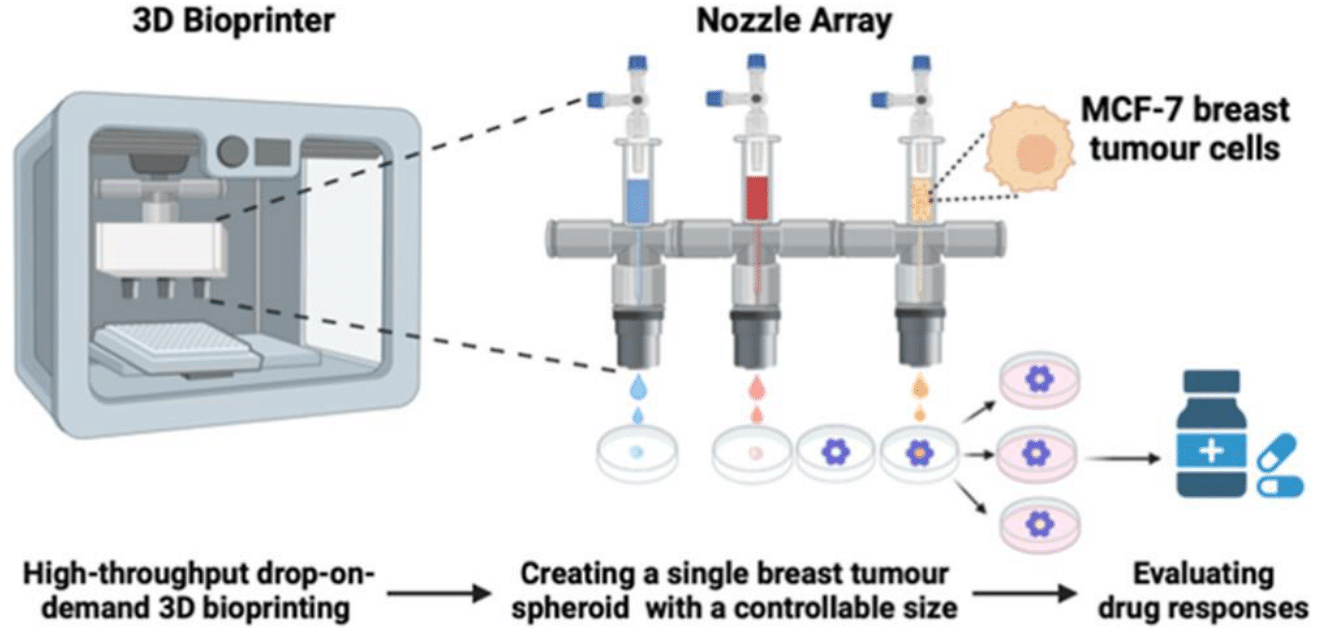
Figure 7 Schematic process for the bioprinting of a breast tumour spheroid from fast-cross-linking bionks.33
The image shows the process of 3D bioprinting breast tumour spheroids through a drop-on-demand bioprinting technique and using fast-crosslinking bioinks.
Another interventional clinical trial, which is currently on Phase 1 and Phase 2, about the utilization of biopolymer-based bioinks integrated into 3D bioprinting technology for the development and implantation of tracheal organs that are specific to each patient have been recently published.34 Lastly, a recent review comprehensively discussed the current contributions of different companies in the evolution of 3D bioprinting, one of which is the bioprinting of collagen-based dermal and epidermal bioinks for vascularized skin substitutes.35 The bioprinted skin grafts, once implanted onto the backs of immunodeficient mice, exhibited exceptional skin regeneration and vascularization.35
Challenges in clinical studies and translation
Several reports identified challenges in the extensive clinical translation of bioinks and bioprinted tissues and/or organs.36,37 Firstly, commercial bioinks typically undergo only basic tests like biocompatibility and post-bioprinting cell viability; however, bioinks may affect other cellular functions in which a bioink might maintain cell viability but not support cell differentiation.36 Moreover, there is widespread debate around testing tissue-engineered organ transplants on healthy volunteers, including challenges in differentiating between the patient's natural response to treatment and the effects of the bioprinted product, alongside issues such as patients inability to withdraw post-implantation.37
Currently, the primary curb in advancing bioprinting technology is the lack of appropriate bioink formulations.22 Moreover, the effective deployment of 3D bioprinting is significantly hindered by the limited selection of bioinks that are both suitable for bioprinting and biocompatible.10 Presently, there are only a handful of bioinks that are capable of bioprinting while accurately mimicking the necessary tissue structure to restore organ function.3
Hence, future advancements in bioink technology are anticipated to focus on creating more sophisticated bioinks that can replicate the complex structure and function of native tissues, ultimately enabling the printing of whole organs.9 Novel approaches to bioink technology, such as the use of recombinant materials, are being developed to enhance the survival, proliferation, and functionality of cell-laden bioinks.38 Advancements are also being made in the printability and biochemical functionality of bioinks, such as the development of microgels/nanogels and other kinds of multifunctional bioinks capable of customizing the local microenvironment to suit specific encapsulated cell types or bioactive factors.1 This presents the potential of bioinks to introduce a new era of personalized medicine, enabling the creation of transplanted tissues tailored uniquely for individual patients.3
Companies:
1a. BICO Group.
1b. Collplant.
1c. Rousselot.
1d. Humabiologics, Inc.
1e. Axolotl Biosciences.
1f. Allevi 3D Systems.
Products
2a. PhotoAlginate®-INK.
2b. X-Pure®.
2c. Gelatin Methacrylate, Lyophilized Beads.
2d. TissuePrint.
2e. Chitoink.
2f. ChiMa.
2g. HumaDerm.
2h. rhCollagen.
2i. Corning® Matrigel® Matrix.
2j. HAMA.
None.
Authors declare that there is no conflict of interest.
None.

©2024 Andrea, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.