Journal of
eISSN: 2572-8466


Review Article Volume 5 Issue 4
Department of Bioorganic Chemistry, Wroclaw University of Science and Technology, Poland
Correspondence: Ewa Zymanczyk-Duda, Wroceaw University of Science and Technology, Faculty of Chemistry, Department of Bioorganic Chemistry, 50-370 Wroceaw, Wybrzeze Wyspia skiego 27, Poland
Received: May 24, 2018 | Published: August 15, 2018
Citation: Zymanczyk-Duda E Szmigiel-Merena B, Brzezinska-Rodak M. Natural antioxidants–properties and possible applications. J Appl Biotechnol Bioeng. 2018;5(4):251-258. DOI: 10.15406/jabb.2018.05.00146
There is an interest in the use of compounds able to prevent organism damages. Antioxidants are such compounds that can protect from cells damages caused by free radicals and may be used in the treatment and prevention on many diseases, such as cancer, cardiovascular disease, diabetes, brain stroke, skin diseases as well as they delay the aging process. There are many sources of antioxidants. They can be synthetic or natural and especially those derived from natural sources, demand special attention. Phenolic compounds are substances which mainly possess such activity, but also vitamins and minerals. There are a lot of antioxidants, but in that review, the chosen compounds with outstanding antioxidant activity, mainly used in pharmaceuticals and cosmetics were described. The review shows the activity of phenolic acids (ferulic acid and caffeic acid) and polyphenols (ellagic acid, curcumin, genistein, hydroxytyrosol, resveratrol) and vitamins (C and E).
Keywords: antioxidants, polyphenols, ellagic acid, ferulic acid, hydroxytyrosol, resveratrol, vitamins
Antioxidants are compounds having ability to scavenge free radicals and inhibit the oxidation of moieties. Free radicals are reactive molecules or atoms containing an unpaired electron and they tend to react with another molecules, to gain electron and stabilize themselves. The formation of free radicals is a risk for all tissues. Free radicals affect lipids, proteins, enzymes, nucleic acids, what leads to damage cells, faster process of aging and moreover cancer disease as a result of destruction of the genetic cell material. These free radicals are formed as a result of action of many factors such as UV radiation, high temperature, environmental pollution, nicotine or drugs.1
Antioxidants can be synthetic or natural and especially those derived from natural sources, demand special attention. There are many phenolic compounds, vitamins and minerals having antioxidant properties. Plant extracts are often used due to the richness of antioxidant compounds. Antioxidants can be administered both orally and topically. They are commonly used in systemic therapy to protect people from the risk of cancer, cardiovascular disease, diabetes, brain stroke, cataract. Moreover, they decrease the effects of aging and protect also against extremely skin-aging. Free radicals lead to the most typical histological and clinical signs of skin photoaging. They affect damage the lipid components of sebum, oxidation of polyunsaturated fatty acids forming the composition of phospholipids in cell membranes as well as destruction of collagen and elastin fibers and hyaluronic acid in dermis. The results are premature wrinkling, pigmentary changes, lack of skin firmness and elasticity, excessive skin dryness as well as a weakness of the immune skin system and increase the risk of skin cancer.2‒4
There is also growing interest for non-sunscreen photoprotective agents. Topical antioxidants are commonly used in combination with sunscreen to enhance their efficacy and offer greater protection to patients. Non-sunscreen materials such as antioxidants may further decrease UV-induced damages compared with sunscreen alone.5
That knowledge about action of free radicals induces to constantly searching for substances able to prevent from many diseases as well as cutaneous damages.
Ferulic acid
Phenolic acids have very good antioxidant potential. Ferulic acid, one of the hydroxycinnamic acids, naturally occurs in many plants. It is commonly found in tomatoes, blueberries, blackberries, strawberries, cereal grains (Figure 1).6‒8
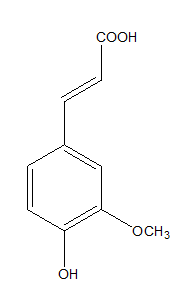
Figure 1 Ferulic acid.9
Ferulic acid may act as antioxidant by different mechanisms such as scavenging reactive oxygen species, metal chelation and inhibition lipid peroxidation.9,10 Moreover, ferulic acid absorbs UV and inhibits inflammatory reactions induced by UV such as erythema, sunburn. Such action plays the role in delaying the skin aging and prevents cancer. Furthermore, ferulic acid stabilizes vitamin C, which makes it more effective.11,12 These all properties make the ferulic acid be used as a health promoter.
Oxidative stress is one of the agent which induces the development of diabetic nephropathy. Experiments showed the antioxidant effect of ferulic acid by decreasing the formation of reactive oxygen species and anti-inflammatory mechanism in rats of type 2 diabetes. Morphological changes in kidneys rats were reduced by treatment with ferulic acid. Therefore ferulic acid may be protective agent in diabetic nephropathy.13
Protective role of ferulic acid on sepsis- induced oxidative damage in the lymphocytes, liver and kidney cells was presented. Protective role of ferulic acid on sepsis- induced oxidative damage in the lymphocytes, liver and kidney cells was presented. The results indicated that DNA damages in sepsis in a ferulic acid-treated group was lower.14
Nicotine plays a significant role in the development of lung cancer through the increasing free radical production. Ferulic acid exerts protective effect against nicotine toxicity and decreases the lipid peroxidation and DNA damages in nicotine-treated rats.15,16 Furthermore, ferulic acid can be used as a potential treatment for cancer via its antiproliferative activity.17 Such actions might be useful in prevention of lung cancer. Ferulic acid shows also cardioprotective effect. The use of ferulic acid in the treatment of cardiovascular diseases resulted in decrease the blood pressure.18,19
Derivatives of ferulic acid also demonstrate an antioxidant activity such as ester derivative of ferulic acid- isopentyl ferulate. Studies revealed that isopentyl ferulate is a good modification of ferulic acid, suggesting that can be used as a new antioxidant agent. Its antioxidant capacity was evaluated by in vitro methods. The radical scavenging ability was assessed by DPPH and ABTS tests. Moreover, an antioxidant capacity against the hydroxyl radical and nitric oxide were also evaluated as well as the inhibition of lipid peroxidation by TBARS method.20
Caffeic acid
Another hydroxycinnamic acid possessing an antioxidant activity is caffeic acid, presents in fruits, wine, coffee and olive oil (Figure 2).21,22
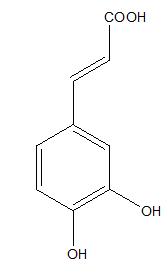
Figure 2 Caffeic acid.9
Caffeic acid exerts a very good radical scavenging effect, which was confirmed in different in vitro antioxidant assays.9,21,23 The antioxidant properties of caffeic acid affect against many global diseases. Caffeic acid indicated the neuroprotective activity on cerebral ischemia-reperfusion injury in rats, which action was likely mediated through the inhibition of 5- lipoxygenase.22
Moreover, the modification of caffeic acid by the addition of glucose could improve its biological properties. Studies demonstrated that enzymatic glucosylation of caffeic acid improved its antioxidant activity in a skin cells models.24
Ellagic acid
Polyphenols have extraordinary antioxidant potential. Ellagic acid, one of the polyphenolic compounds, naturally occurs in various fruits and seeds, such as strawberries, raspberries, grapes, pomegranates. That potential antioxidant can be introduced by diet, pharmaceutical preparations or used as anti-aging agent to external application on the skin (Figure 3).25
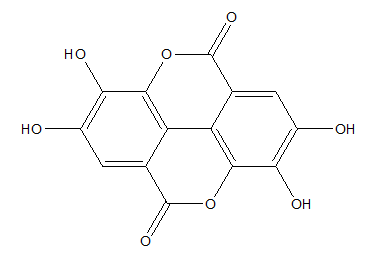
Figure 3 Ellagic acid.26
Ellagic acid demonstrates a great radical scavenging activity. Its antioxidant properties were measured by using the in vitro methods such as DPPH, ABTS, FRAP, ORAC and lipid peroxidation assays. Results indicated that ellagic acid may be useful in prevention of many diseases which are induced by the formation of free radicals.27,28
Liver injuries are often caused by oxidative stress and inflammation reaction. Ellagic acid exerted the protective effect against acute hepatic injury in mice. Results indicated that ellagic acid pretreatment prevent against lipopolysaccharide/D-galactosamine-induced liver injury, by increasing the antioxidative defense system and reducing inflammatory response.29 Ellagic acid protected hepatocytes from damages in mice. That role of relied on prevention vitamin k3-induced reactive oxygen species production and therefore protects against liver cell death.30
Problems with cardiovascular system are often the result of unhealthy lifestyle and the formation of reactive free radicals in organism. Unfortunately, synthetic drugs are commonly used for the treatment of prevention of such diseases. These drugs may certainly adverse side effects. Therefore there is looking for natural compounds showing the therapeutic action.31 Alternative therapies using naturally compounds to protect organism from damages such as heart diseases are becoming popular. The cardioprotective effect of ellagic acid against experimentally induced cardiac arrhythmias in rats was evaluated. Results indicated that oral pretreatment with ellagic acid was effective in cardioprotection and prevented from myocardial infarction. The function of ellagic acid was probably based on inhibition the lipid peroxidation and reduction the concentration of triglycerides and cholesterol in the plasma.32,33 Studies performed on rabbits indicated that ellagic acid could prevent atherosclerosis via suppression of oxidative stress and cell apoptosis.34
Nowadays cancer is frequently occurring disease and a novel strategy for the prevention and treatment are constantly needed. Ellagic acid is able to inhibit the growth of several cancer cells by inhibiting the proliferation. The results indicated the potential antioxidative and chemopreventive effect of ellagic acid. Ellagic acid exhibits potent anticancer activities towards breast, colorectal, ovarian, pancreatic, melanoma and lymphoma cells.35‒39
Skin exposure to UV leads to skin damages such as sunburn, irritations and accelerates the aging process of the skin and induces skin cancer. There is growing interest in the use of natural components in photoprotection. Dietary or pharmacological intervention of ellagic acid delayed the skin aging process induced by UV radiation. Ellagic acid exerted the photoprotective effect on collagen breakdown in UVB-irradiated human skin cells and hairless mice. Ellagic acid prevented from collagen degradation by blocking matrix metalloproteinase production in UVB-exposed fibroblasts. Also topical administration of ellagic acid reduced production of pro-inflammatory cytokines in UVB-exposed hairless mice. That demonstrated that ellagic acid prevents collagen destruction and therefore shows anti-wrinkle activity. Moreover anti-inflammatory properties of ellagic acid lead to its use in prevention of skin photoaging and skin cancer.26 Ellagic acid protected from dermal elastin degradation. Pretreatment with that polyphenol decreased proteolytic degradation of elastin and enhanced it synthesis in aged skin.40
Curcumin
Curcumin is a polyphenol derived from turmeric (Curcuma longa), which is very often used as a food additive (Figure 4).41
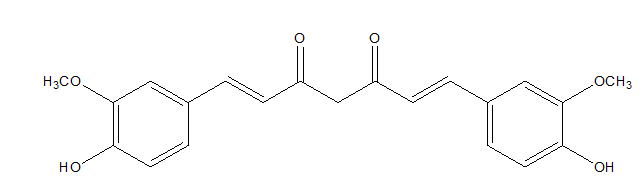
Figure 4 Curcumin.42
Curcumin showed anti-photoaging effect by inhibiting UVB-induced matrix metalloproteinases expression in human dermal fibroblasts, which are responsible for collagen degradation in skin. Moreover, curcumin suppressed the reactive oxygen species formation in fibroblast cells.41,43
The treatment with curcumin indicated essential cardioprotective effects against cardiovascular diseases, which can be induced by high fat diet and obesity. In cardiac H9c2 cells the reactive oxygen species formation, fibrosis and hypertrophy of tissue, as well as apoptosis of cells were suppressed after oral administration of curcumin. The therapeutic effect of curcumin was associated with its capability to activate Nrf2 and inactivate NF-κB.43
The therapeutic activity of curcumin may be hindered by its poor water solubility. Therefore, the complex of curcumin with sulfobutylether-β-cyclodextrin, glucosyl conjugates of curcumin and tetrahydrocurcumin resulted significantly better water solubility as well as showed antioxidant activity.44,45 The novel monocarbonyl analogues of curcumin (MACs) limited myocardial ischemia reperfusion injury through activating Nrf2. First, these curcumin analogues increased its stability, second exerted an antioxidant activity. Studies on H9c6 cells showed the inhibition of oxidant stress parameters. Experiments in animal model indicated the reduction of infarct size and myocardial apoptosis after administration of curcumin.42
Genistein
Genistein is a flavonoid known for modulating activity of estrogens but also characterizes antioxidant and photoprotective activity. Genistein occurs in many leguminous plants but especially in soybeans (Figure 5).46-48
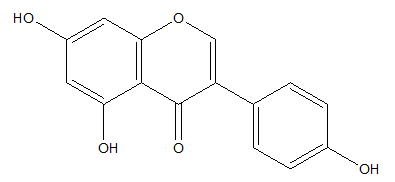
Figure 5 Genistein.49
Genistein showed the inhibition of the processes inducted by UV such as photo-aging and photodamages. In human fibroblasts irradiated with UVB, genistein suppressed activity of senescence-associated beta-galactosidase (SA-β-gal), which is a biomarker of aging. Moreover, genistein reduced the activity of proteins p66Shc and FKHRL1, which are involved in the production of free radicals and consequently oxidative stress.47
That flavonoid is effective in protecting cells against DNA oxidative damages. The activity was also confirmed by the reduction of DNA strand breaks in lymphocytes.50 Genistein ameliorated degenerative changes in cardiac tissue and improved the function of the diabetic myocardium by its anti-inflammatory and antioxidant effects.51 Furthermore its neuroprotective effect in traumatic brain injury was demonstrated, which activity was confirmed by inhibition the disruption of blood-brain-barrier.52 Genistein improved also antioxidant status of kidney in nephrotic syndrome.53
Hydroxytyrosol
Hydroxytyrosol is one of the major phenolic compounds that occurs in olive oil (Figure 6).54,55
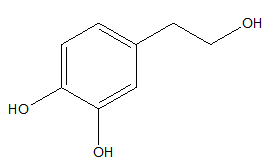
Figure 6 Hydroxytyrosol.56
The main olive component- hydroxytyrosol is an efficient scavenger of several free radicals species such as hydroxyl radicals, superoxide radicals, peroxynitrite.57,58 Hydroxytyrosol indicates anti-proliferative and apoptotic activities which lead to anti-cancer action.59‒61 Studies on human breast cancer on MCF-7 cells models indicated that hydroxytyrosol reduced cell viability, cell proliferation and cell cycle, which led to cell death.59 Anti-cancer effect of hydroxytyrosol on human colon adenocarcinoma cells was exerted through its ability to induce apoptosis by generating reactive oxygen species in colon cancer cells and anti-proliferative activity.60,61
Hydroxytyrosol protects against UVB-induced DNA damages. Research on human skin keratinocyte cell line HaCaT, after exposed them to UVB and treated with hydroxytyrosol, demonstrated the reduction of DNA damages caused by UVB. Furthermore hydroxytyrosol reduced intracellular reactive oxygen species formation caused by UVB.62 Pre-treated human keratinocytes with hydroxytyrosol before UVB exposure also decreased their apoptosis, what is important in protection from skin damages.63 Anti-apoptotic activity of hydroxytyrosol was shown in studies on human monocytic cell line and murine myoblasts cell model. Hydroxytyrosol was the protective agent against H2O2, one of the reactive oxygen species, which induced the apoptotic death in cell lines.64
Oxidative stress is involved in the pathogenesis of various diseases. Hydroxytyrosol can reduce it. It exerted an antioxidant action by inhibiting the production of oxidation products in kidney cells, therefore protected them from damage and renal dysfunction.56 The neuroprotective effect of hydroxytyrosol in mice brain and neuroblastoma cells was also associated with decreased of oxidative stress. That improved the neuron survival after hydroxytyrosol administered.65 Furthermore, hydroxytyrosol exhibited the protective role against oxidative and morphological changes induced by mercury in human erythrocytes. Hydroxytyrosol treatment prevented from hemolysis and reactive oxygen species generation.66
Hydroxytyrosol showed also protective effect in an animal model suffering on rheumatoid arthritis. Hydroxytyrosol supplementation decreased histological damages and improved articular function in treated animals.67 Modifications of hydroxytyrosol may also increase its antioxidant activity. Inclusion a lipophilic chain in the hydroxytyrosol molecule may enhance that activity. Lipophilic hydroxytyrosyl esters showed greater radical scavenging capacity and the protection against protein and lipid oxidation.68 Ester derivatives of hydroxytyrosol penetrate better through human epidermis so they constitute an effective antioxidant for potential topical administration.69 The antioxidant effect of ester hydroxytyrosol laurate carried out on human monocytoid cell line and murine myoblasts cell model resulted in better antioxidant capacity against H2O2 than hydroxytyrosol.64
Resveratrol
Resveratrol, a natural plant polyphenol, commonly occurs in dark grapes, red wine, grapefruits, having strong antioxidant activity as well as inhibits carcinogenesis (Figure 7).70
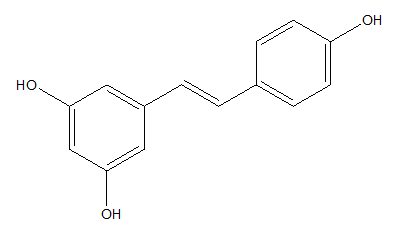
Figure 7 Resveratrol.71
Resveratrol indicates the scavenging DPPH activity and is effective in inhibiting lipid oxidation.72 Moreover, the natural resveratrol dimers such as parthenocissin A, quadrangularin A and pallidol were observed to have better scavenging effects toward DPPH radical than resveratrol monomer.71 Pallidol is a naturally occuring resveratrol dimer from red wine and it demonstrated the strong quenching effects on singlet oxygen.73 The findings showed the potential of resveratrol as a possible agent to reduce lung injury. Resveratrol protected from histopathological changes in the lung during endotoxemia by increasing the anti-oxidant biomarkers. Such activity decreased oxidative stress in endotoxemic lung.74
Next promising effect of resveratrol is photoprotection. Resveratrol showed the chemopreventive effect against UVB-induced damages in hairless mouse skin. Topical pretreatment of resveratrol resulted in inhibition of UVB-induced adverse effects.75 Pretreatment of resveratrol on UVB-treated HaCaT cells resulted in an increase in cell survival. The generation of reactive oxygen species was also attenuated. Therefore, resveratrol attenuated UVB-induced damages and indicated the photoprotective action.76 The derivative of resveratrol- resveratrate, exerted also photoprotective effect on human skin. Resveratrate inhibited erythema on UV-irradiated skin and inhibited sunburn cell formation.77
Furthermore, resveratrol exerts significant cardioprotective effect.78 Studies in rats indicated that resveratrol reduced infarct size and improved ventricular function after myocardial infarction.79,80 Resveratrol decreased myocardial damages by inducing the growth of new capillary vessels and their density, so that maintained the blood flow to the myocardium.80 Besides, resveratrol attenuated ventricular arrhythmias and extended the survival of rats with myocardial infarction.81 Potential cardioprotective activity of resveratrol might be associated with protection cardiomyocytes from ischemia or hypoxia-induced their apoptosis.82 Resveratrol also protected against development of type 2 diabetic vascular disease.83
Vitamin C
Vitamins possess also an antioxidant activity.48,84,85
Vitamin C (L-ascorbic acid), mainly occurs in citrus, but also in wild rose, raspberries, strawberries, cranberries, blueberries. The main activity of vitamin C is to donate electrons and neutralize free radicals. Moreover vitamin C regenerates another antioxidant- vitamin E (Figure 8).84‒87 Vitamin C indicates an antioxidant activity which was confirmed by antioxidants assays (TEAC, FRAP, HOCl).88
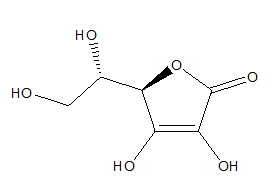
Figure 8 Vitamin C.84
Vitamin C alleviated the inflammation through reducing the levels of inflammatory and metabolic markers such as interleukins, triglyceride, which was demonstrated in hypertensive and diabetic obese adults.89 Topical treatment with vitamin C in photoaged human skin, indicated the improvement of skin hydration, firmness and reduction of superficial wrinkles and skin roughness.90 Vitamin C stimulated the synthesis of collagen and elastin, remodeling elastic fibres in sun-damaged skin as well as prevented erythema and inhibited sunburn cells formation caused by UV exposure.87,90
Vitamin C supplementation in mice showed protective effect against cantharidin-induced liver injuries. Canthadrin is an anti-cancer medication which possesses hepatic toxicity. Also vitamin C demonstrated hepatoprotective activity against chemical liver damage induced by concanavalin A- a lectin. Toxicity within hepatocytes was reduced through inhibition of NF-κB signal pathway.91,92
Vitamin E
Vitamin E is the form of tocopherols and tocotrienols. α-tocopherol is the most biologically active form of vitamin E (Figure 9).84,85
Figure 9 α-tocopherol.84
Vitamin E occurs in wheat, corn, nuts, lettuce, spinach, cabbage. It is one of the main factors that prevents body from free radicals, protects proteins against oxidation, therefore also elastic and collagen fibers in the skin. Due to these antioxidant properties, vitamin E protects the skin from aging.48,84,85,93,94
Topical use of vitamin E provides photoprotection by both antioxidant and UV absorptive properties. The use of vitamin E formulation protected against irritations caused by UV and prevented skin damages.95 Moreover, the combination of vitamin E with vitamin C is recommended due to enhance antioxidant activity. Vitamin C and E provided essential photoprotection for the skin. Taken together in topical formulations, protected from erythema-induced by UV exposure and decreased the number of sunburn cells. Moreover they reduced thymine dimer mutations known to be associated with skin cancer.11
Vitamin E indicated chemoprotective effect in protecting liver tissue from damages caused by cyclophosphamide (CP), which is used in the treatment of cancer or autoimmune diseases. It has serious, life-threatening side effects and toxic effect on liver tissue. Therefore it may be administered with an antioxidant such as vitamin E, which much decreased changes of histological structure of cells apoptosis.96
Furthermore, vitamin E improved the action of chemotherapeutic medications such as Paclitaxel (PTX) or Doxorubicin, which have antiproliferative activity in cancer cells. Anti-cancer activity of PTX against breast cancer was significantly improved with inclusion the vitamin E compare to free PTX. That may improve the chemotherapy of breast cancer by enhance the efficiency and decrease toxicity of PTX. Also, vitamin E loaded with Doxorubicin showed the enhancement of anticancer activity.97,98
Antioxidants are compounds, which exhibit beneficial effect on human health by protection against ultraviolet radiation and free radicals. Human cells are permanently damaged by constant attacks from free radicals which may lead to photocarcinogenesis, immunosuppression and photoaging. That induces a lot of diseases as well as influence adverse on skin condition. Antioxidants prevent from such changes, therefore may be used in the treatment and delay the aging process.3
Phenolic compounds are substances possessing extraordinary antioxidant activity. The most important antioxidants having ability to neutralize free radicals and protect from ultraviolet radiation are polyphenols such as: ellagic acid, curcumin, genistein, hydroxytyrosol, resveratrol as well as phenolic acids: ferulic acid and caffeic acid. Moreover vitamin C and E also exhibit outstanding antioxidant activity. These components with specific activity, different mechanisms of action and possibility of application could be further utilized both in medicine and cosmetic and food industries.
None.
The authors declare that there is no conflict of interest.

©2018 Zymanczyk-Duda, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.