Journal of
eISSN: 2572-8466


Research Article Volume 5 Issue 4
1Department of Health Sciences, Unversita del Piemonte Orientale A. Avogadro, Italy
2Department of Cardiac Surgery, Clinica S Gaudenzio, Italy
3Dipartimento di Scienze del Farmaco, Universita del Piemonte Orientale A. Avogadro, Italy
4Centro di Biotecnologie per la Ricerca Medica Applicata (BRMA), Italy
Correspondence: Maria Prat, Department of Health Sciences, Unversita del Piemonte Orientale A. Avogadro, Via Solaroli 17, 28100 Novara, Italy, Tel 39-0321660662
Received: May 02, 2018 | Published: July 12, 2018
Citation: Antonini S, Colangelo D, Oltolina F, et al. Clovamide protects cardiac progenitor cells from H2 O2 -induced oxidative stress. J Appl Biotechnol Bioeng. 2018;5(4):202-208. DOI: 10.15406/jabb.2018.05.00138
The transplantation of mesenchymal stem cells (MSCs) is a promising approach for tissue repairing, but the survival, engraftment and migration of these cells in the recipient tissue may be compromised by the necrotic and oxidative microenvironment present there. Herein we investigated whether cardiac progenitor cells (CPCs) isolated from human heart biopsies could be protected from an oxidative stress by treatment with clovamide, a natural polyphenolic compound previously isolated in Trifolium pratense (red clover) and cocoa (Theobroma cacao) endowed of anti-oxidant activity. Oxidative stress was induced by short-term H2O2 treatment, and evaluated by ROS production, lipid peroxidation and apoptosis. Treatment of cells with clovamide after oxidative stress showed beneficial effects in terms of reduction of ROS production and of lipid peroxidation, as well as in protection from apoptosis and activation of caspases. These results indicate a possible role of clovamide as protective agent against redox insult. This could positively affect the fate of the transplanted CPCs, which could become more resistant to the hostile microenvironment, and thus show better survival and engraftment.
Keywords: cacao polyphenol, anti-oxidative stress, ROS, cardiovascular disease, cardiac progenitor cells
MSCs, mesenchymal stem cells; CPCs, cardiac progenitor cells; CVD, cardiovascular diseases; hCPCs, human cardiac progenitors cells; IRB, institutional review board; RFU, relative fluorescence unit; LPO, lipid hydroperoxide; SD, standard deviation; ROS, reactive oxygen species; HGFR, hepatocyte growth factor receptor
Regenerative medicine is a new emerging discipline aimed at restoring the functionality of injured tissues and organs, particularly important in cases where the latter contain cells with a low proliferative ability. This is the case of myocardium.1 Cardiovascular diseases (CVD), which are the most prevalent cause of morbidity and mortality world-wide,2 are characterized by a progressive loss of myocytes due to necrosis and apoptosis phenomena. These events are promoted by the oxidative stress associated with the reperfusion that follows the ischemic phase.3,4 The replacement of the lost cardiomyocytes is highly inefficient and relies on the few resident cardiac progenitor cells (CPCs).5 These cells, however, can sustain only organ homeostasis in physiological situations, and, moreover, decline numerically with age1 Heart transplantation is so far the only possible treatment in severe cases, but many problems are associated to this practice, such as insufficient organ availability, complex surgical procedures, costs and long-term immunosuppression. In recent years alternative therapeutic strategies have been suggested, including cellular therapies, mainly based on adult stem cells and trials have started.6,7
However, very few (<5%) of the CPCs directly injected in the heart or in the blood stream can survive.8,9 Several factors are responsible for these deceiving results: namely, the non optimal in vitro cell manipulation, aimed at cell expansion and stemness maintenance, and the subsequent distressing and unfavourable conditions cells encounter once they are transplanted in the injured ischemic myocardium. An unbalanced cellular redox can thus be at the basis of the loss of the transplanted cells.9 The possibility to counteract oxidative stress with molecules endowed of anti-oxidant properties has been advanced and many compounds from the vegetable word, especially polyphenols, have been proposed and investigated.10,11
Polyphenols from Theobroma cacao beans are well characterized for their antioxidant and anti-inflammatory capacity. Flavonoids, a broad family of polyphenols recovered in cocoa (and their derived products) are recognised to be able of protective action towards cardiovascular diseases, as reported by EFSA.12 To obtain the claimed effect, 200mg of cocoa flavanols should be consumed daily. Beside these molecules, other bioactive compounds are present in cocoa as minor component, such as clovamide ((2S)-3-(3,4-Dihydroxyphenyl)-2-[[(Z)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]amino]propanoic acid). This molecule, originally identified in Trifolium pratense13 shows a very interesting anti-oxidant and anti-inflammatory activity in vitro in different systems, such as monocytes, neuronal cells and platelets.14-18
Here in we have investigated the possibility to protect with clovamide, a polyphenolic compound derived from cocoa with anti-oxidant and scavenging activities, CPCs receiving an oxidative stress induced by H2O2.
Cell culture
Human Cardiac Progenitors cells (hCPCs) were obtained from biopsies provided by the Department of Cardiac Surgery of the “Clinica San Gaudenzio” (Novara, Italy) from patients undergoing cardiac surgery after signing a written informed consent according to a protocol approved by the Institutional Review Board (IRB) of Novara (Italy). Samples of right auricola (1-3mm3 specimens) were mechanically minced and enzymatically digested as described by Forte and colleagues.19,20 Partially digested tissue fragments where then plated on 0.02% gelatin-coated dishes in a medium containing 2/3 F12K, 1/3 Claycomb Medium, 10% FBS, 100IU/ml penicillin, 100μg/mL-1 streptomycin and 2mM L-Glutamine (CPC Medium). After 7–14 days, the cells migrating from the fragments were harvested by trypsinization, expanded to obtain approximately 5×106/each biopsy and were sorted by magnetic immunobeads using the anti-Sca-1 antibodies Thermo Fisher Scientific Inc, Monza, Italy). Cells were cultured at 37°C in 5% CO2, changing the medium every other day and passaged when confluent. hCPCs were used from the third until the seventh passage after their isolation and plated at the optimal concentration of 5 x 103cells/cm2.
Induction of oxidative stress
Oxidative stress with H2O2 was induced as described by Pietronave and colleagues with few modifications.21,22 Cells were washed twice in PBS and then incubated in serum-free medium containing 200μM H2O2 for 15minutes, based in preliminary dose-response experiments.
Reactive oxygen species production
To reveal reactive oxygen and nitrogen species production (ROS) within the cells the “Total ROS Detection Kit” (Enzo Life Sciences® (Lausen, Switzerland) was used. Cells were plated on 12mm2 glass coverslips (1x104) coated with 0.1% gelatin 24hours before the experiment. Cells were induced to apoptosis, and then treated with 3μM clovamide for 6hours in complete medium. At the end of the treatments cells were processed following the kit manufacturer’s instructions and mounted, without been fixed, on microscope glass slides with Slow Fade Gold. The kit contained a non-fluorescent, cell permeable dye that react directly with a wide range of reactive species, yielding a green fluorescence. The fluorescent products (excitation at 490nm, emission at 525nm) were visualized using a Leica DMI 6000B fluorescence microscope. For each condition (n=3-5) twenty consecutive fields and, in any case, not less than 150 cells were analyzed. Quantitative analysis was conducted by evaluating cells relative fluorescence unit (RFU) with the graphic software Image J using the ROI Manager function of the Multi Measure plug-in. Clovamide was synthesized as reported in Arlorio et al.23 and the amount used was chosen on the base of preliminary dose-response experiments.22
Lipid peroxidation assay (LPO)
Lipid peroxidation was evaluated using the “Lipid Hydroperoxide (LPO) Assay Kit”. Cells were plated (1x 104) 24hours before the experiment in standard culture condition, induced to apoptosis and treated with clovamide for 6hours as described above. At the end of the treatments cells were processed following the kit manufacturer’s instructions. This kit measures the hydroperoxide directly utilizing the redox reaction with ferrous ions. The resulting ferric ions are detected using thiocyanate ion as the chromogen. The absorbance of each samples (500nm) was red with a spectrophotometer (BioPhotometer plus, Eppendorf). Under the standardized conditions of the assay the dynamic range of the kit was 0.25–5nmol hydroperoxide per sample.
Annexin V/Propidium iodide assay
Cell monolayers (at least 5x104cells) underwent the treatments described above and were then incubated with clovamide for 24h. Cells were then detached with 5mM EDTA, washed twice with PBS and incubated for 15minutes at room temperature with Annexin V–FITC (100nm) and Propidium Iodide (50μg/ml) both from Alexis (Lausen, Switzerland)- diluted in a Hepes/NaOH 10mM, NaCl 140mM, CaCl2 2,5mM (pH 7.4) buffer. Cells were then washed twice with ice cold PBS and fixed in buffered 1% paraformaldehyde, 2% FBS for 15minutes at 4°C and analyzed with FACScan flow cytometer (Becton Dickinson, Mountain View, CA) equipped with a 488nm argon laser.
Polycaspase activity assay
Cells were plated on glass coverslips one day before the experiment. Caspases activity was measured after 15minutes transient H2O2 treatment and a further 6 hours treatment with serum-free medium containing or not 3μM clovamide. hCPCs cells were stained using the Image-iT™ LIVE Red Poly Caspases Detection Kit (Molecular Probes (Eugene, OR)) following the manufacturer’s instructions and mounted on microscope glass slides. Cells were visualized under a Leica DMI 6000B fluorescence microscope equipped with appropriate bandpass filters and an UV lamp for the detection of all three fluorochromes. Twenty consecutive fields for each sample and in any case not less than 150 cells were scored in a double-blind manner.
Statistical analysis
Quantitative analysis is presented as mean± standard deviation (SD) and differences between samples were determined by Student’s t-test. One-way ANOVA and Bonferroni post-test analyses on selected pairs of groups were also performed with Prism (GrapPad software Inc., USA, version 4.03). Values with a p<0.05 or p<0.01 were considered as statistically significant.
Clovamide inhibits ROS production in hCPCs subjected to hydrogen peroxide oxidative stress treatment
We previously showed that a short-term H2O2 treatment were able to induce apoptosis in rat cardiomyoblast (H9c2 cell line) and that the treatment with 3μM clovamide could counteract such an effect.22 We have now extended these studies by examining the activity of clovamide on hCPCs. Here we show that clovamide at a final concentration of 3μM was able to significantly inhibit both the production and the release of reactive oxygen species (ROS) in these cells (93±3%, p<0.001). Figure 1 presents a typical experiment where green intracellular signals associated with H2O2 radical production are detectable (Figure 1).
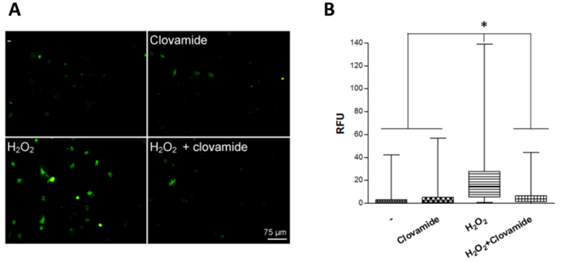
Figure 1 Clovamide inhibits ROS production in hCPCs subjected to hydrogen peroxide oxidative stress treatment. (A) hCPCs cells were plated on glass coverslips and incubated or not (-) with 200μM H2O2 for 15min in serum-free medium, which was then replaced with complete medium containing 3μM clovamide for 2 hours. A representative experiment out of the three performed is shown. (B) Box-plot relative to relative fluorescent unit (RFU) obtained from immuonofluorescence with the graphic software ImageJ. Statistical analyses were performed by comparing cells treated with protective agent vs. untreated cells. *p<0.001 (ANOVA e Bonferroni post-test). All experiments were repeated at least three times in triplicates.
Clovamide inhibits lipid peroxidation in hCPCs induced to oxidative stress
We considered lipid peroxidation as a marker of oxidative damage. When hCPCs were treated with 200μM H2O2 for 15minutes they showed a significantly higher intracellular concentration of hydroperoxides (HP), as compared to the untreated controls (16.3±4.4μM vs 4.2±1.7μM). In contrast, the treatment with hydrogen peroxide followed by treatment with clovamide 3μM for 6hours resulted in a significant reduction in HPs cellular concentration (6.2±1.1μM, p<0.001), leading to HP levels similar to the untreated controls (Figure 2).

Figure 2 Clovamide inhibits lipid peroxidation in hCPCs induced to oxidative stress. hCPCs were incubated or not (Ctrl) with 200μM H2O2 for 15min in serum-free medium and treated with or without 3μM clovamide for 6hours. Hydroperoxide (HP) concentration was evaluated by reading sample absorbance with a spectrophotometer (λ=500nm), using thiocyanate ions as chromogen. Statistical analyses were performed by comparing cells treated with protective agent vs untreated cells. *p<0.001 (ANOVA and Bonferroni post-test). The concentration of HP after the incubation with Clovamide in the sample treated with hydrogen peroxide was similar to the control. The experiments were repeated at least three times in triplicates.
Clovamide protects hCPCs from oxidative stress induced apoptosis
Annexin V-FITC/PI flow cytometry was used to detect the induced apoptosis of hCPCs. Our data show that the treatment clovamide seems to exert a protective activity on hCPCs respect to the untreated control (7.2±2% vs 11.7±2.1%, respectively; p≤0.05). Apoptosis was induced with 200μM H2O2 15minutes treatment, followed by replacement with fresh serum-free medium and incubation for further 24h. This protocol was adopted in order to reproduce some conditions present in acute myocardial ischemia/reperfusion milieu. In this system, the percentage of apoptotic cells after treatment with H2O2 was significantly higher compared to the untreated control (30.2±1.7% vs 11.7±2.1%). The treatment with clovamide 3μM showed an interesting antiapoptotic activity in respect to the untreated control (p≤0.05). When the cells were treated with clovamide after the H2O2 treatment, the molecule was able to significantly abolish the induced pro-apoptotic effect (15±0.7%, p<0.001), as shown in Figure 3 in a representative experiment.
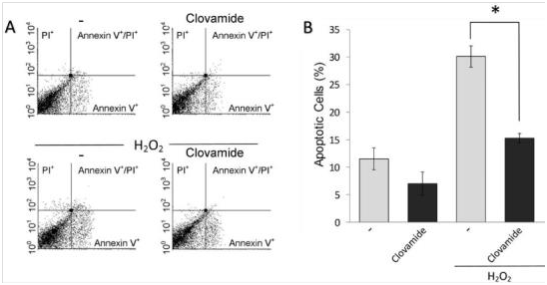
Figure 3 Clovamide protects hCPCs from oxidative stress induced apoptosis. Cells were incubated with H2O2 for 15min in serum-free medium, which was then replaced with complete medium containing or not 3 μM clovamide. After further 24hours incubation cell apoptosis and necrosis were evaluated by labelling with FITC-Annexin V and propidium iodide (PI) respectively. (A) Representative cytograms of such an experiment performed using 3μM clovamide. (B) Graphical representation of the percentage of apoptotic cells (Annexin V-labelled) upon H2O2 treatment and subsequent treatments with clovamide. Statistical analyses were performed by comparing cells treated with protective agent vs. untreated cells (*p<0.001). All experiments were repeated at least three times in triplicates. Clovamide was able to significantly reduce apoptosis induced by H2O2 treatment. All experiments were repeated at least three times in triplicates.
Clovamide inhibits the activation of caspases induced by hydrogen peroxide treatment
Apoptosis usually results from the activation of caspases by the injuring factor. We thus investigated the ability of clovamide to inhibit caspases activation in hCPCs 6h after H2O2 withdrawal. In this assay caspases activation is visualized by the red-fluorescence staining. Figure 4 shows a typical experiment where hydrogen peroxide treatment increased the percentage of cells in which caspases were activated, respect to the untreated control (56.5±3% vs 9.2±2.1%). Clovamide treatment was able to reduce significantly the number of cells in which caspases were activated after H2O2 treatment (56.5±3% vs 7.3±1.1%, p<0.001). Hoechst 33342 fluorescence microscopy was used to qualitatively show apoptosis of cells. Images, at the phase contrast microscope, revealed that cells underwent clear morphological signals of apoptosis, namely cell shrinkage, membrane blebbings and apoptotic bodies upon hydrogen peroxide treatment (Figure 4). Labelling of cells with SYTOX®, a green nuclear dye able to reveal cells with damaged cell membranes, and thus tracing necrotic cells or cells in the late steps of apoptosis, gave negative result.
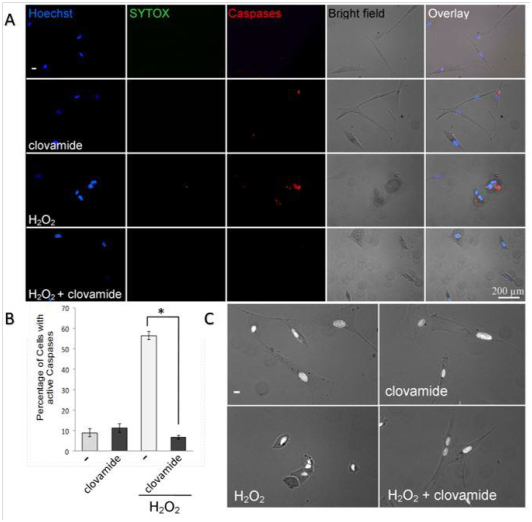
Figure 4 Clovamide inhibits the activation of caspases induced by hydrogen peroxide treatment. hCPCs cells, plated on glass coverslips, underwent transient oxidative stress and further incubation, in complete medium, in the presence or absence of 3μM clovamide. (A) After 6hours, cells were fixed with paraformaldehyde and stained with Hoechst (left column), SYTOX green (second column), SR-DEVD-FMK red poly caspases reagent (third column). Micrographs were merged (fifth column) with bright field images (forth column). (B) Graphical representation of the percentage of cells stained for caspases activation vs total cells. Values were obtained by means of 8 images from 3 separate experiments. Statistical differences among groups were determined (*p<0.001). (C) Morphological aspects of control untreated cells, cells treated with hydrogen peroxide, or clovamide alone, and cells treated with both H2O2 and clovamide. Images revealed, after hydrogen peroxide treatment, clear morphological signals of apoptosis. Cells treated with both H2O2 and clovamide have a normal morphology. The experiments were repeated at least three times in triplicates.
Herein we show that clovamide, a minor component of cacao initially identified in red clover, is able to protect human cardiac progenitor cells (hCPCs) from apoptosis induced by oxidative stress. In fact, clovamide was able to inhibit the production of ROS, the lipid peroxidation as well as the activation of caspases, which are the executers of apoptosis. These data are the development of a previous work performed on the rat cardiomyoblast cell line H9c2, in which we showed that either the activation of the Hepatocyte Growth Factor receptor (HGFR) through an agonist monoclonal antibody21 or the treatment with clovamide22 could counteract the short-term H2O2 induced oxidative stress leading to cell apoptosis. These experiments have been useful to set the best conditions of treatment to be applied to hCPCs, which are somehow more difficult to be obtained, since they must be isolated from auricula of patients undergoing cardiac surgery.19,20,24. The preservation of CPCs potential is a crucial step for their possible use in regenerative medicine.25 The identification of molecules able to protect CPCs against oxidative milieu, typical in hypoxia/reperfusion condition, will give relevant hints for adjuvant therapies in this field. As previously reported, polyphenols are generally considered a broad group of natural compounds ubiquitously expressed in plants, showing antioxidant and anti-inflammatory capacity. Many epidemiological studies have shown associations between a diet rich in polyphenols and protective activity towards cardiovascular diseases.26 Different classes of polyphenols (namely flavonoids), despite a generally low bioavailability, are reported to be able to trigger positive activity in vivo, in animals and humans.
Clovamide, a minor compound present in the antioxidant polyphenolic fraction of Theobroma cacao L. at ppm levels, was originally identified in red clover, and was found also in many other plants.27-31 Its expression can be induced by specific treatment, such as jasmonic acid,32 and, finally, the molecule can be synthetized.16,33-35
Herein we have demonstrated that clovamide treatment of hCPCs could decrease the levels of ROS accumulated within the CPCs after an oxidative stress, which are then responsible for cell apoptosis. ROS, namely O2.-, H2O2, and OH, are produced in biological systems because of the redox reactions and are now considered second messengers, being responsible for the maintenance of the physiological cellular redox balance, governing cellular homeostasis. Indeed, while once they were regarded exclusively as toxic by-products of the cell metabolism involved in tissue injury, nowadays ROS are recognized as active transducers of cell signalling pathways.36 Their levels, low-moderate or excessive, determine if they are beneficial or detrimental for cell survival, respectively.37,38 Because of their critical role in cell functions, ROS levels are controlled both at the level of their production and of their degradation by a series of enzymes.39,40 Moreover, their levels are strictly controlled at a further level through scavenger molecules (both lipophilic and hydrophilic, e.g. vitamin E, anthocyanins).
The possibility to protect CPCs from oxidative stress is particularly relevant, since these cells are extremely rare1, which explains the extremely low regenerative potential of the myocardium, once cardiomyocytes are lost upon an ischemic or non-ischemic insult.41,42
The anti-inflammatory activities of clovamide have been analysed for their potential also in the nervous system, which, similarly to myocardium, is characterized by a very limited regenerative potential and whose cells are thus long-lived. Indeed, clovamide displayed neuroprotective effects in in vitro models of neuronal death by preventing oxidative stress and intracellular Ca(2+) overload, by inhibiting the activation of NFkB and by increasing the expression of peroxisome proliferator-activated receptor-gamma.15 In the nervous system neurodegenerative events are recognized as responsible for Alzheimemr disease and Parkinson disease. Recently, clovamide was reported to be able to inhibit amyloid β aggregation, which characterizes Alzheimer disease.17
Moreover, clovamide was shown to inhibit the production of NO35 and inflammatory cytokines by microglial cells in vitro, and to reduce the expression of glial fibrillary acidic protein (GFAP), a marker of neuroinflammation, to increase tyrosine hydroxylase-positive cells in substantia nigra, and to improve the behavior impairment in a Parkinson's disease mouse model, when administered orally.16,43
Oxidative stress and the associated inflammation has been linked to the pathogenesis also of other diseases,10 such as malignancy,44 kidney diseases,45 diabetes type 2,46 pancreatic β-cell injury,47, fibrogenesis48 which generally results in the apoptosis/loss of functionality of the involved cells. It is thus clear that conteracting oxidative stress can be beneficial for the organism. In the case of myocardium, transplantation of CPCs in the injured organ is a possible therapeutic approach under investigation,6-8 which, however, has encountered many limits, mainly linked to the poor survival/engraftment of the administered cells. Indeed, the number of transplanted cells can decrease to 39% after one hour following intramyocardial injection49 and to less than 5% after 24hours.50 The administration of cell aggregates favours their survival.7,19,51 These cells, more than regenerate the damaged heart, are able to secrete biomolecules that exert paracrine effects and are able to reduce cell death in cardiomyocytes and possibly inducing endogenous CPCs to proliferate.52
Herein we show that clovamide can counteract cell apoptosis and the activity of the caspases, which are their executers. Moreover, clovamide interferes with lipid peroxidation, which, again, cooperates in leading to cell death. Taken together, these data suggest that clovamide is an effective protective agent against oxidative stress in human CPCs and that it could be used in the prophylaxis of cardiac ischemic-reperfusion injury, likely as diet integration component.
Moreover, these encouraging data open to the possibility that this molecule could exert also scavenging activity on hCPCs and thus could be used to empower these cells before transplantation, again enhancing their viability and functionality.
Clovamide, a minor component of cacao initially identified in red clover, is able to protect human cardiac progenitor cells (hCPCs) from apoptosis induced by oxidative stress. In fact, clovamide was able to inhibit the production of ROS, the lipid peroxidation, as well as the activation of caspases, which are the executers of apoptosis. This molecule was already shown to display antioxidant, anti-inflammatory and radical scavenging activities on other cell types, such as human monocytes, neurons and platelets. The demonstration that clovamide can protect hCPCs from oxidative stress is particularly important, since cardiovascular diseases, which are the first cause of morbidity and mortality world-wide, are characterized by a progressive loss of myocytes due to necrosis and apoptosis phenomena, which are promoted by the oxidative stress associated with the reperfusion that follows the ischemic phase. It is expected that clovamide could also empower hCPC or other mesenchymal stem cells in view of their transplantation in the hostile microenvironment of the injured myocardium in the frame of a cellular therapy.
None.
SA and FO carried out the experiments. MP and DC supervised the experiments. DC, MA and MP wrote the paper.
Fondi di Ateneo per la ricerca 2015.
The author declares that there is none of the conflicts.

©2018 Antonini, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.