Journal of
eISSN: 2572-8466


Research Article Volume 3 Issue 4
1Department of Biotechnology and Food Technology, Thai Nguyen University of Agriculture and Forestry, Vietnam
2Division of Molecular Biology, Thai Nguyen University, Vietnam
Correspondence: Duong Van Cuong, Faculty of Biotechnology and Food Technology, Thai Nguyen University of Agriculture and Forestry and Division of Molecular Biology, Institute of Life Sciences, Thai Nguyen University, Vietnam
Received: June 07, 2016 | Published: July 5, 2017
Citation: Nam VH, Trang HHT, Huong TLT, et al. Enhanced ? -carotene biosynthesis in recombinant Escherichia coli harboring the bottom portion of mevalonate pathway of Enterococcus faecium VTCC-B-935 isolated in Vietnam. J Appl Biotechnol Bioeng. 2017;3(4):374-379. DOI: 10.15406/jabb.2017.03.00073
β-carotene is a carotenoid pigment which has commercial value. Biosynthesis of this pigment using recombinant E. coli has been reported. However, improvement of yield is remained necessary. In this case report, bottom portion of mevalonate partway was employed for enhancing metabolic flow to isopentenyl diphosphate, the building block of all carotenoid. Three genes including mvaK1, mvaK2, and mvaD were cloned from Enterococcus faecium VTCC-B-935 isolated in Vietnam and placed into pET28a(+) vector resulted in pET28-K1K2D. Co-expression of this vector with pRSET-lEIBY which contains five enzymes responsible for biosynthesis of β-carotene in E. coli BL21 (DE3) resulted in approximately three fold higher yield of the compound. The high copy number vector pRSET-A showed better performance in production of β-carotene over low copy pET-22b(+). Addition of glycerol significantly enhanced E. coli cell growth and β-carotene biosynthesis. Keywords: β-carotene, enterococcus faecium VTCC-B-935, mvaK1, mvaK2, mvaD,mevalonate pathway
IPP, isopentenyl diphosphate; MEP, 2-C-methyl-d-erythritol-4-phosphate; MVA, mevalonate; DMAPP, dimethylallyl diphosphate; FPP, farnesyl diphosphate; GGPP, geranylgeranyl diphosphate; G3P, glyceraldehyde-3-phosphate
β-carotene, one of the carotenoid compounds, was found mainly in plants. Because of it’s highly antioxidant activity, this compound was shown to have good effects to human health. It is the precursor of vitamin A which is critically required for human.1 Due to good health effects and yellow color, β-carotene is widely used in industries including nutraceuticals, pharmaceuticals, food colorants, cosmetics, and animal feed additives. However, production of natural β-carotene is not sufficient to afford demand of the market. Currently, more than 90% of commercially available β-carotene is chemically synthesized.2 This fact leads a number of research groups to the trend of seeking for alternative sources of natural β-carotene. Natural carotenogenic microorganisms, e.g. Blakeslea trispora, Rhodotorula glutinis, and Dunaliella salina have been used for fermentation to produce β-carotene.3-5
The availability of carotenoid genes from natural carotenogenic organisms leads to another strategy of natural carotenoid biosynthesis. Carotenoid genes were cloned and introduced into non-carotenogenic organism, Escherichia coli, for production of carotenoid, including β-carotene.6-19 E. coli is considered as one of the ideal hosts because of its convenience genetic engineering system and fast growth. However, to date, the production of β-carotene is still unable to support industrial needs because of insufficient yield and stability. Therefore, attempts are required for improvement of production of carotenoid in general and β-carotene in particular. Biosynthesis of β-carotene was affected by a number of factors. In our study, we focus in
Biosynthesis partway of β-carotene is indicated in Figure 1. DMAPP and IPP, the building blocks of carotenoid are synthesized via 2-C-methyl-d-erythritol-4-phosphate (MEP) pathway which is autonomous in our chosen host E. coli. However, in theory the natural yield of these precursors is only sufficient for natural need of E. coli in normal growth conditions which is far less than that required once the organism is used as a microbial factory to produce β-carotene. To address this issue, addition of the exogenous mevalonate (MVA) pathway was shown as a reasonable strategy.6,12,18,20,21 The MVA pathway is divided into two portions, the upper (from acetyl-CoA to MVA) and the lower (from MVA to DMAPP and IPP).
Natural E. coli harbors MEP pathway that enable biosynthesis of FPP from G3P and pyruvate, as well as IPP isomerase catalyze the two-way conversion of IPP and DMAPP, the precursors of carotenoid (yellow part). These precursors could also be synthesized by the mevalonate pathway which is exogenous to E. coli. In this design, the bottom portion of MVA pathway from mevalonate to IPP was recruited from E. faecium VTCC-B-935 isolated in Vietnam (green part). IPP is subsequently used as building blocks for the process of β-carotene synthesis including four steps catalyzed by exogenous crt genes derived from P. ananatis. For better balancing between the two isoforms IPP and DMAPP, in addition to the endogenous idi gene positioned in the genome of E. coli host, another copy of this gene was introduced into the carotenogenic vector for co-over expression with crt genes (blue part).
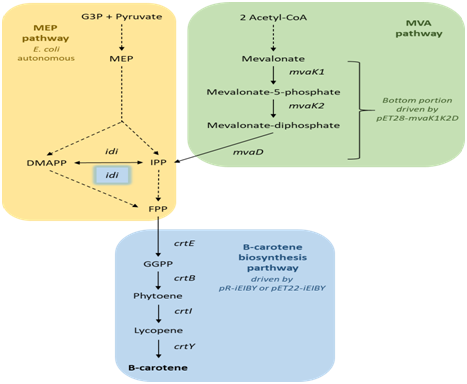
Figure 1 Biosynthesis pathways of β-carotene in recombinant E. coli engineered in this study.
G3P: Glyceraldehyde-3-Phosphate; MEP: 2-C-Methyl-d-Erythritol-4-Phosphate; IPP: Isopentenyl Diphosphate; DMAPP: Dimethylallyl Diphosphate; FPP: Farnesyl Diphosphate; GGPP: Geranylgeranyl Diphosphate
In this case report, we first examine the difference of expression vector backbones. In our previous study, three genes including crtE, crtB, and crtI were cloned from Pantoea ananatis for lycopene biosynthesis, and idi was cloned from E. coli for better balancing of IPP.7 These four genes, together with crtY, were introduced into pRSET-A and pET22b(+) resulted in multicistronic operon vectors pR-IEIBY and pET22-iEIBY, respectively. Secondly, differ from other previous publications, another source of genes encode for the bottom mevalonate pathway enzymes including mvaK1, mvaK2, and mvaD were cloned from Enterococcus faecium VTCC- B-935 isolated in Vietnam and introduced into pET28 vector forming pET28-mvaK1K2D. Subsequently, this vector was transformed into a recombinant E. coli strain which has already contained another expression vector pR-IEIBY. Finally, four additional carbon sources were investigated for higher production of β-carotene using our recombinant system.
Bacterial strains, primers, plasmids, and culture conditions
Bacterial strains, primers, and plasmids used in this study are listed in Table 1. E. coli DH5α was used for molecular cloning of target genes. E. coli BL21(DE3) was used for expression of mevalonate and carotenoid genes and subsequently the expressed recombinant enzymes produced β-carotene. Enterococcus faecium VTCC-B-935 and Pantoea ananatis DSM-17873 were used as donors of target genes. Primer sequences were designed based on nucleotide sequences of target genes available in NCBI database, D90087.2 for crtY and AF290095.1 for mva genes. Backbone plasmids including pLUG, pRSET-A, pET22b(+) and pET28a(+) were used for gene cloning and recombinant enzyme expression.
Cultures of E. faecium and P. ananatis were performed following provider’s instructions. For β-carotene production, recombinant E. coli clones were grown on LB agar plates under selection pressure of ampicillin (100µg/mL) or combination of ampicillin/kanamycin (50µg/mL) in an incubator at 37°C overnight. Individual colonies on plates were inoculated in a flask containing 5 ml of LB media supplemented with the same antibiotic for 12h in a rotary-shaking incubator at 180 RPM. Subsequently the primary culture was transferred into 250mL flask containing 50mL LB media under the same condition. IPTG induction, at different concentrations, was performed while OD600nm reached 0.3-0.5 and continue inoculation for desired periods. Conditions were modified in optimization experiments according to planed changes. For biosynthesis of IPP and DMAPP by bottom portion of MVA pathway, mevalonate was add to the culture at 6.6mM as substrate.13
Cloning of crtY, mvaK1, mvaK2, and mvaD genes
Genomic DNA of P. ananatis and E. faecium were extracted using common protocols. Target genes were amplified using standard protocols and specific primers listed in Table 1. PCR products were subsequently ligated into pLUG cloning vector (Intron Biotechnology) by T4 DNA ligase (New England Biolabs). Ligation products were transformed into competent E. coli DH5α (Thermo Fisher Scientific) cells by heat shock and plated onto LB agar plates supplemented with ampicillin (100 µg/mL), X-gal (20µg/mL) and IPTG (1 mM). White colonies were picked and inoculated in 5 ml LB media. After extraction, plasmids were screened by size separation by agarose electrophoresis, and then mapped by appropriate restriction enzymes. Target inserts in positive clones were sequenced by Marcogen. Gene sequences were in silico analyzed by Vector NTI software to ensure correct translation, and aligned to available database using BLAST.
Name |
Description |
Reference/Source |
Bacteria Strains |
||
E. coli DH5α |
TA cloning host |
Thermo Fisher Scientific |
E. coli BL21(DE3) |
Expression host compatible with vectors driven by T7 system |
Thermo Fisher Scientific |
Pantoea ananatis DSM-17873 |
Carotenoid genes donor |
German Collection of Microorganisms and Cell Cultures |
Enterococcus faecium VTCC-B-935 |
Mevalonate genes donor, isolated in Vietnam |
Vietnam Type Culture Collection |
Primers* |
||
mvaK1-F |
ggatccATGGCAAACTATGGCCAAG |
This study |
mvaK1-R |
gaattcTTAAACATAGGTATGTACTCCT |
This study |
mvaK2-F |
gaattcATGATTGAAGTATCTGCACCA |
This study |
mvaK2-R |
gagctcTCATCGGTTTTCCTTTCTTTGA |
This study |
mvaD-F |
gagctcATGTTTAAAGGCAAAGCACG |
This study |
mvaD-R |
gcggccgcTTATTCAATAATCGCAATTCCTG |
This study |
crtY-F |
TgaattcAGGAGGTGTCTTAAATGGGAGCGGCTAT |
This study |
crtY-R |
GCaagcttTTAACGATGAGTCGTCATAATGG |
This study |
Plasmids |
||
pLUG |
TA cloning vector |
Intron Biotechnology |
pET28a(+) |
Expression vector for low copy number (pBR322 ori), strong phage promoter (T7), IPTG induction (lac operon), and Kanamicin selection (Kanr) |
Novagen |
pET22b(+) |
Expression vector for low copy number (pBR322 ori), periplasmic localization (pelB signal peptide), strong phage promoter (T7), IPTG induction (lac operon), and Ampicilin selection (Ampr) |
Novagen |
pRSET-A |
Expression vector for high copy number (pUC ori), strong phage promoter (T7), IPTG induction (lac operon), and Ampicilin selection (Ampr) |
Thermo Fisher Scientific |
pR-iEIB |
pRSET-A containing idi of E. coli and crtE, crtI and crtB of P. ananatis |
[7] |
pR-iEIBY |
pRSET-A containing idi of E. coli and crtE, crtI, crtB, and crtY of P. ananatis |
This study |
pET22-Y |
pET22b(+) containing crtY of P. ananatis |
This study |
pET22-iEIBY |
pET22 containing idi of E. coli and crtE, crtI, crtB, and crtY of P. ananatis |
This study |
pET28-K1 |
pET28a(+) containing mvaK1 of E. faecium |
This study |
pET28-K1K2 |
pET28a(+) containing mvaK1 and mvaK2 of E. faecium |
This study |
pET28-K1K2D |
pET28a(+) containing mvaK1, mvaK2, and mvaD of E. faecium |
This study |
Table 1 Bacterial strains, primers, and plasmids used in this study
(*) Template binding sequences are indicated by underlined letters, start and stop codons are bold, and restriction sites are lowercase. In cases of no restriction sites over hanged, autonomous sites in the carrying vectors were used instead
Construction of recombinant plasmids
In our previous study, four genes including idi, crtE, crtI, and crtB were introduced into pRSET-A resulted in pR-IEIB which was used for biosynthesis of lycopene.7 In this study, cloned crtY gene was excited from pLUG-crtY and inserted into pR-IEIB using HindIII restriction enzyme resulted in pR-IEIBY. Subsequently, the multicistronic operon in pR-IEIBY containing five genes was moved to pET22b(+) by two steps. First, only crtY was moved using EcoRI and HindIII resulted in pET22-Y. Second, the fragment containing four genes idi, crtE, crtI and crtB was moved to pET22-Y using XbaI and EcoRI resulted in pET22-iEIBY. Similar experimental procedure was performed to introduce mvaK1, mvaK2, and mvaD into pET28a(+) to construct pET28-K1, pET28-K1K2, and pET28-K1K1D at the positions of BamHI/EcoRI, EcoRI/SacI, and SacI/NotI, respectively. All vectors constructed in this study are listed in Table 1 and the maps of three major vectors are presented in Figure 2.
Where, pR-EIBY and pET22-IEIBY are pRSET-A and pET22b(+) containing multicistronic operon of five genes including idi from E. coli DH5α, crtE, crtI, crtB, and crtY from Pantoea ananatis, respectively. These two vector were used for biosynthesis of β-carotene in recombinant E. coli BL21(DE3). pET28-K1K2D is pET28a(+) containing multicistronic operon of three genes including mvaK1, mvaK2, and mvaD from Enterococcus faecium VTCC-B-935. This vector was used for conversion of mevalonate to IPP. Major component of the vectors and restriction sites used in the construction were indicated. Vector maps were drawn using Vector NTI software version 11.5.1.
Measurement of β-carotene production
Concentration of β-carotene synthesized by recombinant E. coli was measured by HPLC according to the protocol previously described by Yoon et al.12 with slight modifications. E. coli cells were harvested by centrifugation at 12,000 rpm and washed once by ddH2O. Cell pellet was re-suspended in 1mL of acetone and incubate at 55°C for 10 to 15 minutes in dark condition. The extracted acetone containing β-carotene was collected by centrifugation at 12,000 rpm for 15 minutes. HPLC analysis was performed on the Agilent 1260 system using Zorbax C18 column (250 mm×4.6 mm, 5 micromet) with detector at 454 nm. Two mobile phases were used including methanol (phase A) and acetonitrile (phase B). Isocratic program (A:B= 70:30) and flow rate of 1ml/min was used to all analyses. Standard β-carotene was purchased from Sigma (Cat.No. C4582).
Comparison of pRSET-A and pET22b(+) for biosynthesis of β-carotene in engineered E. coli
Different expression vectors produce different levels of desired recombinant proteins; in this case the five enzymes involved in the biosynthesis of β-carotene, and subsequently affect production of this carotenoid pigment. Therefore, we first compare two of the most common commercially available expression systems including pRSET-A and pET22b(+). Although these two vector systems are both driven by T7 promoter, they are significantly different in term of copy number. In case of carotenoid production by recombinant E. coli, contradictory observations regarding the biosynthesis efficiency caused by plasmid copy number were reported. Jones et al.22 showed equal or better performance of low copy plasmids in comparison to high copy plasmids.22 However, using a mutated version of the low copy vector pDC318 that made it a high copy one, Tao et al.23 observed that mutated vector showed higher level of β-carotene biosynthesis.23 In our study, accumulation of β-carotene was higher with pRSET-A than pET22b(+) according to higher density of yellow color of the cell pellet (Figure 3). HPLC analysis confirmed the biosynthesis of β-carotene by pR-IEIBY and pET22-iEIBY are 5.5mg/L and 3.1mg/L, respectively. pRSET-A harbor pUC origin of replication which manages approximately 500 copies of the vector per single cell. This number is about 10 to 20 folds higher than what driven by pBR322 origin of replication located in pET22b(+). Although pET22b(+) and pRSET-A are not totally identical but they are both driven by T7 promoter. Therefore, in term of plasmid copy number our data is in agreement with Tao et al.23
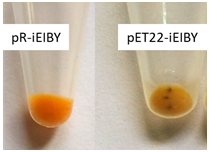
Figure 3 Comparison of pRSET-A and pET22b(+) for biosynthesis of β-carotene in recombinant E. coli BL21(DE3).
Higher accumulation of β-carotene in cell pellet of pR-IEIBY than in pET22-IEIBY was demonstrated by higher density of yellow color which is the color of this pigment.
Increased production of β-carotene by addition of bottom portion of mevalonate pathway
Isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) are precursors of all carotenoid, including β-carotene. Therefore, to enhance production of β-carotene, it is reasonable to direct the biosynthesis flow of the E. coli host toward these building blocks via either endogenous MEP pathway or exogenous MEV pathway, or both (Figure 1). Several studies have proven the feasibility of this strategy.6,12,13,18 In genetic engineering studies focusing on production of recombinant proteins, it is common that different sources of target genes resulted in different level of expression because of the suitability between genetic code of foreign genes with the genetic reading system/mechanism of specific host. In this study, we test another set of bottom portion of MVA pathway derived from E. faecium isolated in Vietnam.
E. coli BL21 (DE3) harboring pR-IEIBY was made competent. This enabled second transformation with pET28-K1K2D resulted in a recombinant strain harboring both carotenoid vector and MVA vector, in this case are pR-IEIBY and pET28-K1K2D, respectively. E. coli culture was supplied with mevalonate at 6.6 mM which the optimal concentration is shown by Yoon et al.13 It is clear that addition of bottom portion of MVA pathway of E. faeciumVTCC-B-935 did not affect growth of E. coli (Figure 4A). This observation is in agreement with several previous reports.6,11-13 To date, β-carotene has not been shown as a toxic agent to E. coli. As expected, over expression of three mva genes significantly up regulates the biosynthesis of β-carotene (Figure 4B). At 48 hours and 96 hours of cultivation, addition of bottom MVA pathway derived from E. faecium VTCC-B-935 resulted in 12.6mg/L and 17.7mg/L of β-carotene which are approximately three folds higher than 4.2mg/L and 5.6mg/L, respectively, produced in control strain. This result indicated that the three genes, mvaK1, mvaK2, and mvaD of E. faecium VTCC-B-935 are comparable to the corresponding genes of Streptococcus pneumoniae in term of IPP biosynthesis in recombinant E. coli .13
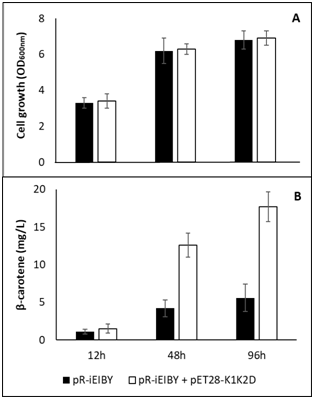
Figure 4 Cell growth and β-carotene production of recombinant E. coli with or without addition of bottom portion of MVA pathway from E. faecium VTCC-B-935.
pR-IEIBY contains β-carotene biosynthesis genes and idi. pET28-K1K2D contains genes encoding enzymes catalyze reactions from mevalonate to IPP.
Effect of addition of carbon sources on β-carotene production
Carbon source was determined as one of the factors related to biosynthesis of β-carotene. Insufficient availability of carbon sources restricts the growth of cell. Again, contradictory observations were reported regarding the effect of different carbon sources on biosynthesis of carotenoid by recombinant E. coli, including β-carotene. Biosynthesis of toulene was shown increased and decreased following addition of glycerol and glucose, respectively.24 Contradictorily, production of lycopene was significantly enhanced by addition of glucose in high cell density fed-batch fermentation conditions.25 In this study, four sources of carbon were investigated, including glucose, galactose, maltose, and glycerol. The optimal concentration is 0.5% according to Yoon et al.12 Therefore, we added 0.5% of each of carbon sources to the 2YT medium. In Figure 5, the positive correlation between cell growth and β-carotene yield was observed. Among the tested carbon sources, glycerol indicated significantly improvement in both cell growth and β-carotene production over the others. In this case, the highest yield of β-carotene was 65 mg/L which was approximately three fold higher than the control which did not contain additional carbon source. Beside glycerol, addition of maltose slightly improved cell growth and β-carotene production. However, addition of glucose and galactose resulted in lower cell growth and β-carotene biosynthesis compared to the control. One of the reasons to explain this phenomenon is that glucose and galactose decreased pH of the culture that leads to inhibition of E. coli growth.
The bottom portion of mevalonate pathway of E. faecium VTCC-B-935 isolated in Vietnam was introduced into a engineered carotenogenic E. coli resulted in improvement of β-carotene biosynthesis. The pRSET-A expression vector performed better than pET22b(+). Addition of glycerol as carbon source enhanced E. coli cell growth and biosynthesis of β-carotene almost 3 folds.
This work was supported by the Research Grant from the Vietnam Ministry of Education and Training (Grant No B2014-TN02-01).
The author declares no conflict of interest.

©2017 Nam, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.