Journal of
eISSN: 2572-8466


Review Article Volume 3 Issue 3
Obducat Technologies AB, Sweden
Correspondence: Rizgar N Jiawook, Obducat Technologies AB, Lund, Sweden
Received: May 09, 2017 | Published: June 7, 2017
Citation: Jiawook RN. Manufacturing nanoimprint lithography system to produce efficient microneedles patch for transdermal drug delivery. J Appl Biotechnol Bioeng. 2017;3(3):321-324. DOI: 10.15406/jabb.2017.03.00065
Transdermal drug delivery device is a sophisticated device for delivering drugs continuously via the skin to the systemic circulation in the human body. Advanced technologies needed to manufacture at low costs in high volumes. Nanoimprint Lithography (NIL) system was manufactured for the first time by Obducat AB to make this possible. The system comprises of the NIL process to imprint a pattern on a foil with nano structure mimics human cell structure. This foil covers the microneedles and then followed by the laser cutting of the nano structured foil to fit onto the microneedles patch. This results a very efficient Microneedles Patch for Transdermal Drug Delivery.
Keywords: nanoimprint lithography, transdermal patches, microneedles, drug delivery, transdermal devices
NIL, nanoimprint lithography; EHDA, electro hydrodynamic atomization; TDD, transdermal drug delivery; SEM, scanning electron microscopy; AFM, atomic force microscopy
Syringes and hypodermic needles have been used to deliver drugs to patients for more than 160 years. These syringes are painful when injected through the human skin to deliver the drug into the systemic circulation. An alternative method for delivering drugs was introduced namely Transdermal drug delivery patches. The Transdermal Drug Delivery Patch is a method to deliver a liquid drug into the blood stream through the skin without pain. Some drugs are mixed with solutions to ease the penetration of the drug that is placed in the patches. Transdermal patches are adhesive patches placed on the skin in order to deliver a specific amount of drug dose that will penetrate the skin to reach the blood stream. These patches were developed in the 1970s and first approved by FDA in 1979. Nowadays, skin patches are used for motion sickness, quitting smoking, angina, to relieve the pain of shingles, etc., which has been discussed in Patel et al.1 These patches have limitations; it delivers only small drug molecules mixed with a solution such as alcohol to ease penetrating the skin. Technology advancement in the field of micromechanics led to the invention and manufacturing of the microneedles. Microneedles can deliver small and large drug molecules, the microneedles penetrate the hard skin surface (Stratum layer), which was the barrier for not letting large drug molecules to pass through. The microneedles make holes big enough in the Epidermis layer of the skin and the drug is delivered to the systemic circulation directly. The sizes of the holes made by the microneedles are in micrometers depending on the drug molecule size.
Transdermal drug delivery patch types
There are four types of the Transdermal Drug Delivery patches
In all these types explained above and the newly developed Vapor patches, the drug molecules must be smaller than the pathway between the adjacent cells of the skin. For larger drug molecules, a new method has been developed using microneedle patches, see (Figure 1). The Microneedles physically disrupts painlessly the outer layer of the skin (Stratum Corneum), which is the greater barrier to delivering most drugs. Therefore, Microneedles can deliver higher molecular weight and water soluble drugs through the skin to reach the systemic circulation.
The largest organ in human body is the skin. The area of the skin in adults is about 1.5m2, which provides protection for all other internal organs as demonstrated in Lhernould et al.2 The main layers of the skin are Epidermis, Dermis, and Hypodermis (Subcutaneous). The Epidermis layer encompass the Stratum Corneum (the outer surface of the skin), and it is of thickness 10-15 micrometers. The thickest part of the skin is the Dermis about 4 millimeters. It contains blood vessels, nerves, hair follicles, oil glands; see Jepps et al.3 and Andrews et al.4 The third layer Hypodermis (Subcutaneous) is the Adipose tissue (fat). Figure 2 shows a detailed skin cross section of the human body. The Transdermal Drug Delivery Device main objective is to deliver drugs into systemic circulation of the human body through the skin at a predetermined rate with no human intervention. Also, it reduces the load off the digestive tract and liver and pain of the hypodermic needles when injected into the skin.
Transdermal drug delivery devices are sophisticated systems for delivering drugs via skin to the systemic circulation in the human body. Transdermal drug delivery device needs advanced technologies to manufacture at low costs in high volumes. We have manufactured an industrial NIL system to produce efficient microneedles patch using easy process in high volumes, fully automated, and cost effective. The emergence of micro-fabrication manufacturing technology made it possible to utilize the microneedles for delivering small and large molecules through the Stratum Corneum of the skin to reach the dermis layer and deliver the appropriate drug. Microneedles Transdermal Drug Delivery device is a sophisticated device from the manufacturing point of view. This type of Transdermal Drug Delivery device has been developed to deliver large drug molecules as well as small drug molecules through the human skin continuously to the systemic circulation in the human body. The microneedles make micron sized bores in the skin to deliver the drug across the dermis layer. This will help patients with needle phobia to apply the patch by them without pain as demonstrated by Donnelly et al.5 Figure 3 shows the different shapes of the microneedles.
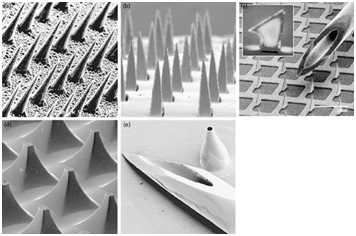
Figure 3 Shapes of microneedles used for transdermal drug delivery compared with the conventional syringe.
Microneedles types
Microneedles can be divided into four types: solid, coated, dissolving and hollow as demonstrated by Jepps et al.3 and Andrews et al.4 Figure 4. The solid microneedles are used to make bores (micro channels) in the skin, thereafter, a drug patch is applied on the created micro channels. Gupta et al.6 suggests that the drug patch should be applied instantly to prevent undesired infection by pathogenic microorganisms. The coated microneedles are the solid microneedles coated with a drug. Many techniques for preparing coated microneedles have been suggested in the literature. One of these techniques is electro hydrodynamic atomization (EHDA) for preparing smart microneedles as its demonstrated by Khan et al.7 This technique has been used to prepare nano- and micrometer scaled pharmaceutical coating on stainless steel of height 600-900 micrometers. Based on this technique by making some variants in the coating process, they manage to deposit particles 100 nanometers to 3 micrometers and fibers 400 nanometers to 1 micrometer directly on microneedles in a selectable way. Dissolving MN is convenient for patients, because it is a one-step application. They are fabricated from polymers using micromoulding method. It is fabricated by pouring polymer in its solution state into a structured female master for the microneedles arrays under vacuum or pressure to make the micro cavities of the MN arrays then filled with the solutions of the drugs and dried. The encapsulated drug will be released at the time the patch attached to the skin. This method has been investigated by Wang et al.8 The hollow microneedles look like hydrodermic needles but much shorter. The drug liquid is infused through the bores in the microneedles into the skin.

Figure 4 Types of microneedles used for transdermal drug delivery.
Imprinting nanostructured foil
Walsh et al.9 disclosed a method to make the microneedles more efficient in delivering drugs and reducing the human body immune system response to the drug. Nanoimprint Lithography (NIL) system made this possible in advanced and more efficient manufacturing process. We have realized the first such system and delivered to the customer; see Jiawook et al.10,11 The developed NIL system imprints a patterned thin foil with nano structure similar to the human cell structure on the foil Figure 5 and Figure 6, which covers the microneedles, then followed by the laser cutting of the nano-structured foil to fit onto the microneedles device. This process insures friendly reaction of the body cells to the external compound because of the imprinted structure that covers the surface of the microneedles mimics’ human skin cell structure. This technology has been described in the patents Ross.12,13 Figure 7 shows the microneedles arrays chip, and the enlarged microneedles arrays in Figure 8.
The replication of the nano structure using NIL technology that mimics cell structure is imprinted on a few microns’ thick foil accomplished by Soft Press® technology. Figure 9 shows the microneedles arrays chip (top left), microneedles arrays enlarged (middle left), SEM image of the imprinted nanostructured foil (bottom left), and the tip of the one enlarged microneedle covered with the nano structured foil (right). The manufactured transdermal drug delivery (TDD) device in this process has the following advantage, the immune system does not respond to the TDD device, it is painless, the device TDD showed that the efficiency improved x200 times, and the device is sufficiently flexible and deformable that permits the device to maintain agent-transmitting relationship upon skin such as sensitive areas. The Figure 10 bellow shows the imprinting section of the NIL system that imprints the nano structured foil mimicking the skin cells on the foil, also shows a 6x6 chips tray holding the microneedles arrays chip. The foil imprinting and Laser cutting section of the NIL system where the foil is cut after it covers the microneedles arrays to fit the chip size.
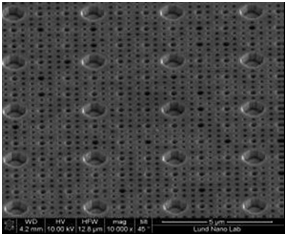
Figure 5 SEM (Scanning Electron Microscopy) Image for the mold used for cell physical structure like pattern.
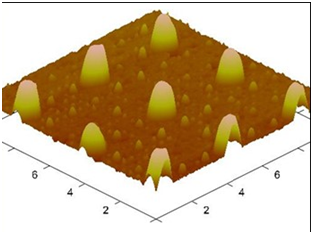
Figure 6 AFM (Atomic Force Microscopy) Image for the Imprinted foil with replicated pattern using the mold.
The outer look of the manufactured Nanoimprint Lithography system shown in Figure 11. This system houses the rollers system to manage the foil roll and the polymer stamp material roll, the nanostructures imprinting section, the laser cutting section of the foil to fit onto the microneedles arrays chip size, the robot section that handles the microneedles chips from and to the tray, and the NIL system interface screen. The software for handling the operations of the NIL system was developed internally at Obducat AB.
We at Obducat AB have realized the manufacturing of a NIL system. This system insures high throughput production of imprinting human skin cell structure on few micrometers thin foil covering the surface of the microneedles to have friendly reaction mimics’ skin cell structure. This transdermal drug delivery device has advantage over the naked microneedles by which; the human immune system does not respond to the device and the drug, it is painless, the device showed that the efficiency improved x 200 times, and it is sufficiently flexible and deformable that permits it to maintain agent-transmitting relationship upon skin such as sensitive areas.
None.
The author declares no conflict of interest.

©2017 Jiawook. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.