Journal of
eISSN: 2572-8466


Research Article Volume 2 Issue 5
1Process Engineering and materials Lab, France
2Department of Planktonology, University of Sfax, Tunisia
Correspondence: Hela Ben Amor-Ben Ayed, Process Engineering and materials Lab, Centrale Sup?lec, Grande Voie des Vignes, 92295 Ch?tenay-Malabry, France, Tel 33695246886
Received: November 04, 2016 | Published: March 24, 2017
Citation: Ben Ayed HBA, Taidi B, Ayadi H, et al. The use of Chlorella vulgaris to accumulate magnesium under different culture conditions. J Appl Biotechnol Bioeng. 2017;2(5):180-185. DOI: 10.15406/jabb.2017.02.00043
We investigated magnesium (Mg2+) ion uptake by Chlorella vulgaris under mixotrophic growth conditions (10g/L of glucose) in a stirred photo-bioreactor. The culture nitrate and glucose consumptions were followed and analyzed during the experiments. The cellular chlorophyll α concentration decreased during exponential growth, indicating an adaptation to heterotrophic metabolism. The partition of magnesium partitioned between the culture medium, the cell surface and within the cells was determined throughout the experiment. A clear relationship between the microalgal concentration and Mg2+ ion removal-extent from the medium was observed. The removal rate was faster during mixotrophic growth than autotrophic growth and was related to the higher biomass production under the latter culture conditions. The Mg2+ concentration per gram of dry biomass was 3.44mg/g under heterotrophic conditions and 6.0mg/g under autotrophic growth. At the end of the experiment (330h), 90% of the initial magnesium (17.7mg/L) in the medium was associated with the biomass, of which 4% was adsorbed and 86% absorbed by cells. This study was consistent with the kinetic model based on a reversible first-order reaction for Mg2+ bioaccumulation in C. vulgaris. Mixotrophic growth conditions can be used at industrial scale to increase both the speed and the extent of Mg2+ uptake by the microalgal.
Keywords: chlorella vulgaris, mixotrophy, magnesium, intracellular absorption, extracellular adsorption
Mg2+, magnesium; NO3-, nitrate; A800, absorbance measured at a wavelength of 800nm
The autotrophic green microalgae C. vulgaris can grow heterotrophically if presented with a suitable organic carbon source1-3 or mixotrophically by simultaneous autotrophic and heterotrophic metabolisms.2,4 C. vulgaris can use a number of organic carbon sources such as glucose or acetic acid. Glucose and other organic substrates can stimulate the growth of this alga in the presence or the absence of light.5-7 Microalgae cultures offer a wide industrial application due to their ability to assimilate metals.8,9 The intended application of this study is to enrich C. vulgaris with magnesium for cosmetic purposes. We have previously reported that C. vulgaris is not inhibited by up to 500 mg/L Mg2+ ions and that the alga could accumulate a relatively large amount of Mg2+.10,11 This metal is essential for photosynthesis as it occupies the core of the chlorophyll molecule and influences the activity of various photosynthetic enzymes.12 The large Mg2+ accumulation amount renders C. vulgaris of industrial interest for the recovery/elimination of useful metals such as magnesium from aqueous media. One aim of this study was to examine the way in which C. vulgaris grew under mixotrophic growth conditions by providing it simultaneously with two carbon and energy sources ; light, CO2 and glucose. A second aim was to investigate the magnesium accumulation (extracellular and intracellular) under these conditions and to further investigate the validity of the previously reported model for the Mg2+ biomass-association under autotrophic conditions.10
Microorganism and growth medium
The strain Chlorella vulgaris CCAP211/e 11B (trebouxiophyceae) was obtained from the Culture Collection of Algae and Protozoa, CCAP (UK). The growth medium used was the modified 3N-Bristol medium,13,14 in which the sole nitrogen source is nitrate at a concentration of 547.03 mg/L. The glucose concentration of 10 g/L was added to the medium prior to sterilization. The base Mg2+ ion concentration was enriched to approximately 18 mg/L in order to compare with previous autotrophic culture of C. vulgaris at the same Mg2+ concentration.11
Bioreactor cultures
C. vulgaris was grown in a cylindrical stirred tank “Biostat B plus” bioreactor (5L working volume; Sartorius, Germany). Stirring (750 rpm) was provided by a three palettes (UniVessel 5L), each inclined at 45°C from the horizontal axis (pitch blade stirrer) (Figure 1). The total light intensity at the inner surface of the bioreactor was measured (LI250A, LI-COR, USA) at 2900 µmol/m2.s achieved by 6 LED lamps (IKEA LEDARE, 2700 Kelvin, 27°C dispersion angle, France). The culture temperature was maintained at 25°C by circulating water through a jacketed vessel (Figure 1). Continuous aeration with sterile air (0.2 µm Sartorius gas filter) at 500ml air/min (1 atm. 25°C) was used to supply both oxygen and CO2 to the culture. The inlet and outlet airflow rates were measured daily. The sterile medium was inoculated with a shake-flask (50 ml in 250 ml flask) culture of C. vulgaris at the end exponential growth (72-96h). The inoculum volume constituted 1% (v/v) of the bioreactor working volume (5L), which gave a cell concentration of 2 × 106 cells/ml at the beginning of the experiment. The inoculum was prepared in the same medium as that used in the bioreactor (MBM medium containing 10 g/L of glucose).
Analytical techniques
Growth measurement: Three methods were used to measure the biomass concentration: absorbance at 800 nm; cell count; dry weight biomass concentration. The cell number per liquid volume was determined by counting the cells using a microscope (Carl Zeiss axioplan imaging 2, Germany) and a Thomas counting chamber of 0.1 mm depth. The absorbance (A800) of the algal cultures was measured at 800 nm (Spectrophotometer Cary 300 Scan UV-visible, Varian Inc, Netherlands) after appropriate dilution. The cell viability was measured using a GUAVA easyCyte™ flow cytometer (Merck Millipore, France) with the ViaCount (14-0155) method. The dry weight (DW) biomass concentration (g/L) was determined by the following method. The culture sample was first centrifuged (10 minutes, 1800 g). The pellet was washed through re-suspension in an equal volume of deionized water and centrifuged again (10 minutes, 1800 g). The resulting pellet was transferred into a dry pre-weighted ceramic thimble and heated (24 h, 105°C) in an oven. After drying, the thimble was weighed. In order to follow the growth rate, three samples (10-15 ml) were removed daily from the bioreactor during the exponential growth phase (0-74 h) and only one sample a day was analysed in the other growth phases.
Determination of the glucose concentration: Glucose consumption was followed by measuring glucose concentration in the culture medium (supernatant) obtained from all the samples. This measurement was performed with a High-Performance Liquid Chromatography (HPLC) (Ultimate 3000, column: HPX-87H). A total pressure of approximately 870 psi at 45°C was applied. The eluent was sulphuric acid (2 mm). All samples were first filtered through 0.2 µm and C18 columns to retain all particles and proteins that could influence the HPLC analysis.
Determination of the nitrate concentration: The NO3- analyses were performed by ionic chromatography using a Dionex AS11-HC column installed in a Class 10.000 clean room under the following conditions: system Dionex DX-500, column (CR-ATC, P/N 060477) (4 mm) with a guard column AG11-HC and a total pressure of 2.300 psi at 30°C, conductimetric detector CD20. The eluent (KOH) concentration was 30mmol/L at 1.5ml/min.
Determination of the chlorophyll concentration: Triplicate samples were removed daily from the bioreactor in order to extract and calculate the cellular chlorophyll α concentration during the experiment according to method of Porra.15
Determination of the magnesium ion concentration: An Atomic Absorption Spectrophotometer (Hitachi Z-2300, Japan) was used in order to measure the Mg2+ ion concentration using an air-acetylene flame and by measuring the absorbance at a wavelength of 202.6 nm with the specific magnesium lamp (Agilent technologies, France). The standard deviations for the calibration curve (0.15 to 20.0 mg/L) were in the range 1 to 2 %. The sample dilutions were carried out with Milli-Q water acidified with HCL (pH< 3) to prevent metal precipitation. The calibration solutions were prepared from 1000 mg/L magnesium stock solution (Fischer scientific) and measured 5 times in order to determine the precision of the analysis.
Estimation of adsorbed and absorbed magnesium concentrations: The concentrations of adsorbed (extracellular) and absorbed (intracellular) magnesium were determined by the procedure described by Franklin et al.16 and Ma et al.17 with minor modifications as published in our previous work.10
Magnesium bioaccumulation modeling: A reversible reaction of first order with respect to the magnesium concentration in the culture liquid was assumed for Mg2+ ions uptake by cells. This model has been already published with all details.16 The model is given by the following Equation (1):
Equation (1)
Where k.a is the kinetic constant, the concentrations C, C0 and Ceq are the Mg2+ ion concentrations at times: t, t0 and at equilibrium respectively (mg/L).
Algal growth characteristics in a stirred photo-bioreactor
C. vulgaris was grown independently and in duplicate in stirred photo-bioreactor cultures under mixotrophic conditions in order to investigate magnesium accumulation. Each experiment lasted 310h. The initial glucose and Mg2+ ion concentrations were 10 g/L and 17.7 mg/L in medium, respectively. The absorbance A800, the cell concentration Nb (number of cells/ml) and the dry weight concentration DW (g/L) were measured daily except during the exponential growth phase where they were measure three times per day. These three growth parameters showed a satisfying degree of proportionality for the duplicate experiments (Figure 2), and confirmed the results of Rocha et al.18 “Corrected absorbance” refers to the absorbance measured with diluted samples and multiplied by the dilution factor. The following correlations were obtained:
A800 = (3.3 × 10-8) Nb (cells/ml) and
DW (g/L) = (1.0 × 10-8) Nb (cells/ml).

Figure 2The relationships between the different measurement methods of C. vulgaris biomass: corrected absorbance and dry weight concentration were plotted against cell concentration. The standard deviations of measurements for both experiments are presented with error bars.
The dry weight of an average individual C. vulgaris cell was calculated and found to remain constant at 1.0×10-11g throughout the experiments, which corresponds to published values for this algal.19 Cell viability remained at 96± 2% throughout the experiments. The growth curves under both autotrophic (765h) and mixotrophic (310h) culture conditions are presented in the same Figure 3. The specific growth rate (µ) for the culture was determined from the absorbance data during the exponential growth phase at 0.056h-1 according to the method of Wood et al..20 The µ value was relatively close to the published values (µ= 0.06h-1; Perner Nochta et al.21) for the same organism in a batch cultures in tubular bioreactor. The cell concentration after 310 h of culture was higher (A800= 23 corresponding to 8.8 × 108cells/ml) in mixotrophic growth conditions than the biomass concentration obtained (A800=5 corresponding to 2 × 107cells/ml) after 765 h of autotrophic culture in the photo-bioreactor (Figure 3). These results are in good agreement with Lee et al.22 which investigated the mixotrophic growth (glucose 18g/L) of Chlorella sorokiniana) and reported a higher cell concentration compared to autotrophic growth. For mixotrophic culture conditions, glucose and nitrate (NO3-) concentrations were measured (Figure 4).
Figure 4 shows that no significant consumption of glucose was observed during the exponential growth phase; this demonstrates autotrophic growth during this growth phase. The end of the exponential growth phase coincided with nitrate exhaustion, suggesting a limitation of growth by nitrogen. Three growth phases can be observed: exponential, transition and stationary phases. During the transition phase, glucose was rapidly removed from the growth medium suggesting mixotrophic growth. The exhaustion of glucose corresponded approximately to the start of the stationary phase as the cell concentration became stable. C. vulgaris exhibited a preference for autotrophic growth as long as nitrogen was available but after complete nitrogen exhaustion, glucose consumption occurred. There were slight differences in glucose consumption and cell growth between the duplicate experiments during the transition phase; elsewhere the repeatability between the experiments was very high. The average cellular chlorophyll α content (mg chlorophyll α per g DW) decreased sharply during the exponential phase and remained approximately constant at a low value afterwards (Figure 5). This could be explained firstly by the decreasing of nitrates concentration in the medium that influence the chlorophyll production and secondly by the change of cell physiology upon glucose metabolism. This behavior was also observed by Illman et al.23and Li et al.24 for the same microorganism in mixotrophic cultures. In this study, after exponential growth, the cellular chlorophyll content decreased to a constant value of 5× 10-11 mg/cell. According to Kong et al.25 a high glucose concentration (>5 g/L) inhibited chlorophyll production.
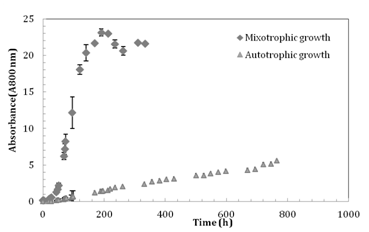
Figure 3 Growth curves for both experiments under mixotrophic and autotrophic growth17 conditions. Corrected absorbance versus time of incubation.

Figure 4 Glucose and nitrate concentrations profiles during C. vulgaris growth. The standard deviation of measurements is presented in the Figure with error bars.
Dissolved Mg2+ ion concentration
The dissolved and biomass-associated Mg2+ ion concentrations (adsorbed and absorbed) were measured early on (74h), in the middle (170h) and at the end (310h) of the experiment. Each measurement was performed in triplicate. From the dissolved Mg2+ ion concentration the removal yield from the growth medium was calculated and plotted against the time of culture (Figure 6). A relationship emerged between Mg2+ ion removal from the medium and the biomass growth. Magnesium removal continued even after the end of the exponential phase until almost total removal (Figure 6). Mg2+ ion uptake continued during the heterotrophic growth phase; the uptake of this ion did not seem to be only related to chlorophyll synthesis. Mg2+ ion is also implicated in the activation of glycolytic enzymes for glucose metabolism.26
Biomass associated magnesium ions
The adsorbed and absorbed (extracellular and intracellular) Mg2+ ion increased during the experiment (Figure 7), the amount of absorbed Mg2+ ion was always greater than the amount that was adsorbed. By the end of the experiment(310h), 90 % of the initial Mg2+ ion content (17.7 mg/L) of the medium had been taken up by C. vulgaris, of which 4 % of the total Mg2+ ions had been adsorbed on the cell wall and 86 % had been absorbed into the cells. The calculated magnesium mass balances accounted for 97±2.0 % of the initial amounts of the ion (Figure 7). The calculation of the proportions of magnesium associated with biomass (adsorbed and absorbed) compared to the initial concentration added in the medium is subtracted from the mass balance as follows:
(Mg)initial: The initial magnesium concentration added in the culture medium.
(Mg)supernatant: The magnesium concentration dissolved in the medium and not captured by biomass.
(Mg)intracellular: The magnesium concentration absorbed into the microalgal cells.
(Mg)extracellular: The magnesium concentration adsorbed on the cells walls.
(Mg)samples: The magnesium concentration of the samples removed from the culture. All magnesium concentration were expressed in mg.

Figure 7 Repartition of Mg2+ ions in the biomass during growth. The mass balance deviation refers to the amount of Mg2+ ions that could not be accounted for. The error bars show the difference in the results observed between the two experiments.
Compared with our previous publication,11 the accumulation of magnesium by algae in mixotrophic culture was lower than that in autotrophic culture. At the end of the experiment (310h), C. vulgarisabsorbed on average 3.44 mg of Mg2+ ions per g of dry biomass whereas in autotrophic photo-bioreactor cultures (765h), 6.0 mg were absorbed per g of dry cell weight. In terms of volumetric productivity and efficiency of accumulation from the medium, of course the mixotrophic conditions provide and advantage. It is then effective strategy to use autotrophic followed by heterotrophic conditions to produce more biomass and to maximize Mg2+ ion uptake. As an interpretation, the Mg2+ bioaccumulation mechanism is as a result of a coupling between biochemical reactions and mass transfer. The external transfer of Mg2+ from the solution to the algae depends essentially on the hydrodynamics and the biochemical reactions largely depend on the cells physiology and the growth limitations (light). A greater extent of growth would present a larger surface area though which transfers could occur.
Model for Mg2+ ion accumulation by C. vulgaris
The experimental results were plotted versus the predicted model curve (Figure 8). They fitted well with the exponential model for magnesium uptake by C. vulgaris confirming its applicability. These results were in good agreement with our previous published work,10 where we developed an exponential model for the Mg2+ ion uptake by C. vulgaris in shake-flask autotrophic cultures with different initial Mg2+ ion concentrations (8.9-465 mg/L). Two differences were found with our previous work.10,11 firstly Ceq seems to be negligible and secondly Mg2+ ion uptake starts after a lag time of 45 h from the start of the experiments. This shows that the majority of the ion is accumulated while growth occurs under heterotrophic conditions. In other words, the exchange surface produced by the greater growth brought about by heterotrophic growth dominated the Mg2+ ion adsorption and absorption phenomena. This model described well the Mg2+ ion uptake by C. vulgaris. Future experiments will be performed to explore Mg2+ ion uptake under different glucose and initial Mg2+ ion concentrations and to find suitable explanations for the different behaviors observed in this study.
The biomass production of C. vulgaris under mixotrophic conditions was significantly higher than in autotrophic cultures. The nitrate exhaustion brought about the end of the exponential phase and it coincided with the start of glucose consumption; there was nevertheless no direct evidence of true mixotrophy where glucose and CO2 would be incorporated into the biomass simultaneously. C. vulgaris adopted an autotrophic metabolism before the exhaustion of the nitrogen source even in the presence of glucose. The specific growth rate was 0.056 h-1 close to that reported by Perner Nochta et al. 21 at 0.058h-1 for the same strain in a batch tubular bioreactor culture. The chlorophyll α was an indicator of photosynthetic activity and was followed during the experiment. Starting with an initial Mg2+ ion concentration of 17.7 mg/L in the growth medium, C. vulgaris accumulated 3.44 mg of Mg per g of dry biomass under mixotrophic growth and 6.0 mg of Mg2+ per g of dry biomass under autotrophic growth. Mg2+ ion removal by biomass from growth medium was directly related to the cell concentration and physiology. During autotrophy, the cells need magnesium to synthesize chlorophyll for photosynthesis, so they accumulate a larger amount of magnesium. In contrast, during heterotrophic growth less Mg2+ is required by the cells as they switch to using glycolysis and respiration.
The micro-organism showed a preference for autotrophic growth even in the presence of glucose. Later on during the experiment, there was no evidence for mixotrophic growth although this could not be excluded neither. The duplicate experiments were highly reproducible, throughout the entire duration of the experiments (310h) and for all the different parameters measured. This study supported our previous results10,11 that C. vulgaris is a suitable microorganism for Mg2+ ion uptake and the method first published by Franklin et al.16 is applicable for the measurement of Mg2+ ion uptake in C. vulgaris. The experimental data fitted well with the exponential model for Mg2+ ion uptake by C. vulgaris cells. The work presented here confirms the application of this model for metal ion sorption in microalgae cultures and the modeling work will be further pursued as in additional experiments under different conditions. This study presents promising results for the development at industrial scale of C. vulgaris cultureswith high cell production and Mg2+ ion uptake potential.
The authors wish to thank Soliance SAS, the CentraleSupelec Chair for Industrial Biotechnology and the Tunisian Ministry of Research and Higher Education for financial support. In addition, the authors would like to thank Barbara Malinowska and Vincent Butin for their technical assistance with the ion chromatography and atomic absorption analyses. Thanks to Clément Hussenet for his assistance with the glucose measurements. Thanks to Cyril Breton and Thierry Martin for the construction and assembly of various laboratory equipment.
The author declares no conflict of interest.

©2017 Ben, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.