Journal of
eISSN: 2572-8466


Review Article Volume 2 Issue 3
1Federal University of Santa Catarina, Brazil
2University of S?o Paulo, Institute of Biosciences, Brazil
Correspondence: Bill Tawil, UCLA, 420 Westwood Plaza, Room 5121, Engineering V, P.O. Box 951600, Los Angeles, CA 90095-1600, USA
Received: January 01, 2016 | Published: February 24, 2017
Citation: Lobos J, Batish A, Sharma A, et al. Home-grown kidneys: challenges and mechanisms behind tissue engineering for the treatment of chronic kidney disease. J Appl Biotechnol Bioeng. 2017;2(3):78-86. DOI: 10.15406/jabb.2017.02.00029
Chronic kidney disease (CKD) is the leading cause of death for individuals suffering from end-stage kidney failure. While kidney transplantation is the most viable solution to CKD, it has many challenges and limitations. This review evaluates kidney transplant rejection and the various transplantation methods that may be employed to treat CKD. Kidney rejection and long-term transplant survival is the main concern regarding transplantation attempts. Thus, scientist must screen and strategize appropriately to ensure kidney transplant survival. Tissue engineering techniques are explored as means to improve upon transplantation attempts. Research into ensuring adequate organ scaffold design has become crucial in the field of regenerative medicine. Furthermore, the role of integrins is discussed as a central component for future tissue engineering techniques, by taking the study of scaffold design to the molecular level. Finally, a glimpse into the current advancements of three-dimensional bioprinting highlights the future of tissue engineering and shows how the field of regenerative medicine is taking another step closer to developing “home-grown” kidneys. With the prospect of generating a fully lab-created, personalized kidney, CKD may one day be treated without encountering any of the limitations that have hindered previous attempts.
Keywords: home-grown, kidney transplantation, integrin, bioprinting, tissue engineering
CKD, chronic kidney disease; PEG, polyethylene glycol; PCL, polycaprolactone; CAPs, cell adhesive proteins; DDR, discoidin domain receptors; LIFT, laser-induced forward transfer; CAD/CAM, computer-aided design and manufacturing; CKD, chronic kidney disease; MtoBS, multiple-head tissue/organ building systems; ATG, antithymocye globulin; BKV, BK virus; CMV, cytomegalo virus; DM, diabetes mellitus; EBV, epstein-barr virus; MMF, mycophenolate mofetil; mTOR, mammalian target of rapamycin; CAP, cell-adhesive peptide, ESP, enzyme-sensitive peptide, GF, growth factor
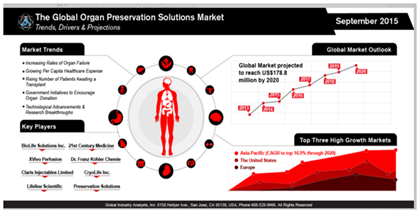
Market size
In the United States, approximately 400 billion dollars is spent annually on the treatment of patients who are suffering from end-stage organ failure, representing 50% of total healthcare costs.1 According to Global Industry Analysts, Inc., it is expected that the market for organ preservation will reach 178.8 million US dollars by the year 2020 (Figure 1).2 This market is showing a steady increase due to the high rate of end-stage organ failure, organ shortage and long wait times for organ transplantation.2 The scarcity of available organs and organ donors is becoming a major problem as the need for organs increases.3 As of January 2016, there are 100,791 individuals in the United States are waiting to receive a kidney transplant.4 Currently, there are only 13,066 available organ donors, leaving 87% of patients without an available organ.5 According to the United States Department of Health & Human Services, an organ recipient is being added every ten minutes to the waiting list and twenty-two patients die every day waiting for an organ transplant.5 The large gap in supply and demand has even led to some organ donors seeking monetary compensation on the black market.6 Moreover, even when a viable organ is available, it may be unusable due to lack of consent.7 Although there is much awareness regarding the need for organ donors, individuals are unable to donate their organs unless they have provided written consent.8 Although organ transplantation is the most viable option for treating CKD, it is clearly not a perfect solution.9,10
Kidney transplantation methods
When a CKD patient is ready to receive a new kidney, there are still many factors that come into play when considering the best path to successful treatment. In general, there are three types of kidney transplantation.11,12 Iso-transplantation occurs between genetically-matched individuals, allo-transplantation occurs between non-genetically matched individuals and Xeno-transplantation occurs between two different species.11,12 The lack of sufficient donor kidneys has lead to xeno-transplantation as a possible solution.13 Most researchers consider pigs to be the most suitable source of organs due to the vast supply of available animal tissue.14 Moreover, xeno-transplantation has the added benefit of being able to manipulate the organ to specifically match the recipient’s needs.14 Yet despite its potential, xeno-transplantation is still considered by many to be an unsafe form of transplantation. Xeno-transplantation carries a high probability of organ rejection and transmission of infectious agents from the donor tissue.15 Lastly, the shorter life span of donor animals means that their organ would age at a faster rate than a human organ.12 Iso and allo-transplantation remain safer alternatives over xeno-transplantation but they are still not guaranteed solutions, as they are still capable of eliciting an immune response to the donor tissue.16 It is essential to monitor organ health to ensure long-term survival following transplantation.16 To reduce the probability of organ rejection, the donor is screened for compatibility with the recipient’s body.11 If compatibility is not ensured beforehand, the kidney will be rejected.11
Kidney transplant rejection
Rejection mechanism: Rejection typically occurs when a transplant recipient contains antibodies against human leukocyte antigen (HLA) molecules expressed in the donor tissue.17 The presence of many different HLA alleles makes it difficult to always obtain donor/recipient compatibility.17 HLA incompatibility leads to both cellular and antibody-mediated immune responses.18,19 A cellular-mediated immune response is more prevalent and occurs either through direct or indirect recognition.18,19 During direct recognition, T-cells are activated by interacting with foreign HLA molecules on the surface of donor antigen presenting cells (APCs).18,19 With indirect recognition, T-cells become activated by donor HLA molecules bound to the recipient’s own APCs.18,19 Additionally, different kinds of cytokines (such as interleukin-4 and interleukin-2) are secreted by activated CD4+ T cells.19 The secretion of cytokines leads to the initiation of immune response including inflammation, activation of pro inflammatory cells and proliferation of T and B cells.19 While the exact mechanisms of rejection are not yet fully known, what is known is that the production of these cytokines and other alloantibodies cause activation of the complement system.20-22 Activation of the complement system causes inflammation and further activation of the immune system, thus leading to damage of the transplanted organ.20-22
Types of rejection: There are three types of organ rejection, which are dependent on the speed of rejection following organ transplantation hyperacute, acute and chronic.23 The main cause of hyperacute rejection is the presence of recipient alloantibodies because of a previous transplantation, blood transfusion, pregnancy, or infection.11 These alloantibodies lead to the activation of the complement-mediated response 24 which prevents vascularization of the transplanted tissue.17,25 Once the kidney has been rejected by the recipient’s body, it must be removed immediately.17,25 Hyper acute rejection can take place within the first few hours following surgery and typically not after 24 hours.11,12 Acute organ rejection is the most common, occurring within the first three months following transplantation; however, there may still be a risk of chronic rejection occurring several years later.12,24 Chronic rejection is characterized by induction of fibroblast proliferation, pleomorphic effects (such as proliferation of smooth muscles) by some cytokines, maturation of B-cells and antibody synthesis by IL-4.26-28 Moreover, fibrosis formation is very common with chronic rejection due to the activation of the tissue repair process.29,30 Immuno suppressants are administered to transplant recipients to hinder the immune response and allow for survival of the transplanted tissue 31 Although immune suppressants are extremely necessary, they also carry some adverse effects (Table 1) ,32 immune suppression reduces the patient’s ability to fight against communicable diseases, enhances the risk of metabolic complications and may elicit toxicity to the immunosuppressive drug.31 The questionable success of kidney transplantation has raised the desire for alternative treatment methods.
Drug |
Cardiovascular toxicity |
Malignancies |
Selected infections |
Bone marrow suppression |
Other |
||||||||
Hypertension |
Dyslipidaemia |
DM |
CMV$ |
EBV* |
BKV+ |
||||||||
Glucocorticosteroids |
++ |
+ |
++ |
.* |
N |
Cushingoid appearance, sleep disturbances, mood changes, impaired wound, healing, osteoporosis |
|||||||
Azathioprine |
N |
N |
N |
+$ |
+ |
Hepatotoxicity |
|||||||
MMF |
N |
+ |
+ |
N |
. |
+ |
+ |
Gastrointestinal symptoms |
|||||
Cyclosporine |
++ |
+++ |
+ |
+ |
+ |
Nephrotioxic, neurotoxic, gum hyperplasia |
|||||||
Tacrolimus |
+ |
++ |
++ |
+ |
+ |
+ |
Nephrotioxic, neurotoxic, gum hyperplasia |
||||||
mTOR-inhibitors |
N |
+++ |
+ |
. |
. |
+ |
Impaired wound healing, flulike syndrome, acne |
||||||
ATG |
+ |
+ |
+ |
++ |
Cytokine release syndrome |
||||||||
Alemtuzumab |
. |
+ |
+ |
Mild cytokine release syndrome, induction of auto immune disease |
|||||||||
Rituzimab |
. |
. |
+ |
Infusion reactions |
|||||||||
Basilizimab |
. |
+ |
+ |
Hypersensitivity reactions |
|||||||||
Belatacept |
++ |
++ |
+ |
+ |
+ |
||||||||
Bortezomib |
. |
+ |
Neurotoxicity |
||||||||||
Eculizumab |
+ |
Very expensive |
|||||||||||
Table 1 Adverse effect of immunosuppressant drugs.32
*Used in many treatment protocols for malignancies; however, an increased risk of malignancy has also been described; $historically associated with an increase risk of malignancy, when azathioprine was given in high dosages. The risk association for currently used lower dosages is less clear; $CMV infections are increased with all immune suppressive medications; however, mTOR inhibitors are thought to decrease the risk, where as ATG increases the risk; #EBV increase the risk of PTLD (Post transplant lymphopoloferative disorder). Induction or rejection therapy with polyclonal and monoclonal antibodies increases the risk of PTLD. PTLD can be treated with rituximab; $the risk of BKV infection is mostly dependent on the total load of immune suppression, but MMF and tacrolimus appear to increase the risk.
ECM scaffolds for organ tissue engineering: Organ tissue engineering is a growing field with great potential to resolve major health problems by combining materials science and medicine. The ultimate goal of tissue engineering is to create functional constructs that can restore function and structure to damaged tissues or whole organs.33 The approach relies on using biocompatible materials to create a scaffold for cellular growth and promote tissue regeneration.33 These scaffolds must provide support for proper cell adhesion and proliferation.34 Thus, the main role of the biological scaffold is to create architecture for cellular growth that mimics the natural extracellular matrix.34 By adding appropriate growth factors and creating the proper conditions, cells can grow on these scaffolds and regenerate the tissue that was previously damaged.34 The key requirements for a successful biological scaffold are: topographical characteristics that mimic native tissue, a porous framework that allows cell and nutrient permeability, non-toxic biodegradability, biocompatibility with natural tissues and an economical manufacturing process.35 Table 2 illustrates the main types of scaffolds used in tissue engineering and how they differ.36
Type |
Examples |
Advantages |
Limitations |
Natural |
Fibrin. Collagen, Alginate |
|
|
Decellularized |
De-cellularized kidney matrix |
|
|
Synthetic |
PEG, PLGA |
|
|
Table 2 Comparison of scaffolds used for tissue engineering.36
Decellularized scaffolds: Decellularized scaffolds provide an excellent option for whole organ engineering, as they are specifically tailored to the organ.37 The decellularization process involves the complete and non-disruptive removal of cellular material from a particular tissue, thus isolating a naked matrix.37 In doing so, a scaffold for cellular growth is derived that is conducive to cellular growth and has a low chance of eliciting an immune response from the recipient.37 Physical, chemical and enzymatic methods can all be employed to create the matrix.37 While the goal is to produce a preserved ECM architecture that is ideal for tissue remodeling, most methods of decellularization disrupt the ECM to some degree.37 The main goal is to try and maintain the native ECM skeleton during the removal of the native biomolecules, as any leftover molecules can hinder constructive remodeling by eliciting an immune response.38 Many factors can affect the integrity of the decellularized ECM scaffold, such as tissue density and morphology.38 The decellularization process typically involves mechanical agitation of tissues along with some form of chemical treatment.38 Mechanical disruption may involve freeze-thaw cycling or high pressure in the presence of detergents to break apart the cells from the underlying matrix.38 Increased pH can prominently aide cell removal but it may also affect the ECM constituents.38 Detergents are effective in their ability to solubilize cell membranes and detach DNA from proteins.38 The percentage of protein removed from the ECM varies with respect to different factors including exposure time, tissue type and age of donor tissue.38 Extra care should be taken when employing acids, bases and detergents as they can also damage nucleic acids and proteins in the ECM.38 Triton X-100 and SDS are typical options that have been used to create decellularized kidney scaffolds.38 Enzymatic disruption is also quite common, but again holds the possibility of damaging the structure and porosity of the matrix with prolonged exposure.38 Nevertheless, once the underlying organ scaffold has been fully decellularized, it can be re-seeded with appropriate cells to begin the tissue regeneration.36
Natural scaffolds: While decellularized scaffolds hold great potential for whole organ engineering, they do not truly address the problem of organ shortage, as organs must still be available to generate these scaffolds.36 Typically, these come in the form of cadavers or xenografts.36 The greatest limitation here lies in biocompatibility of the xeno organ and also the potential for disease states being carried across.36 A better solution comes in the form of other naturally-occurring matrices that can be created in the laboratory.36 Fibrin scaffolds serve as a common favorable matrix for tissue engineering as they satisfy most of the key requirements.39 Fibrin acts as a perfect natural matrix for cell proliferation that can be easily tuned by adjusting the concentrations of the thrombin and fibrinogen co-factors.39 However, while natural scaffolds offer great versatility and biocompatibility, they are limited in structural strength and longevity.36 Thus, natural scaffolds are often conjugated with synthetic polymers to compensate for these limitations.36
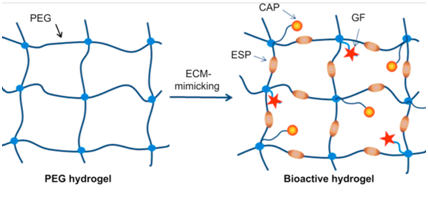
Synthetic scaffolds: The most prominent benefit of synthetic scaffolds lies in their programmable reproducibility 36 (Table 2). In this characteristic alone, synthetic scaffolds hold the potential to resolve the issue of organ shortage.36 The ability to program scaffold creation with reproducible precision, using readily available synthetic polymers, becomes especially evident with the innovation of automatic manufacturing via 3D bioprinting.36,40 Synthetic hydrogels such as polyethylene glycol (PEG) or polycaprolactone (PCL) can be used either alone or in tandem with other scaffolding biomaterials to produce an ideal ECM for cellular growth.36,40 Studies have shown how gelatin or elastin can be incorporated to decrease biodegradability and improve structural strength.35 Additionally, growth factors and enzyme-specific peptides can also be incorporated to influence biodegradability and matrix permeability in a similar way to previously discussed natural scaffolds 36,39,40 (Figure 2). This ability to control and manipulate the architecture of synthetic scaffolds, as well as tune biodegradability and strength, has made synthetic scaffolds more favorable over natural ones in many applications 35,36,40 (Table 1). Yet while these scaffolds satisfy most of the key requirements mentioned earlier, they lack the necessary factors to fully ensure proper cell attachment and proliferation .40 Synthetic scaffolds must be supplemented with key proteins and growth factors in order to promote proper cell adhesion 37 (Figure 2). The incorporation of cell adhesive proteins (CAPs) and growth factors transform synthetic scaffolds like PEG into a matrix that is more mimetic of naturally-occurring ECM 40 (Figure 2). In addition, the incorporation of peptide sequences in synthetic scaffolds has become a prominent modification to help facilitate cell adhesion 40 Further studies into the mechanisms behind cell attachment has elicited an interest in a key factor that may prove pivotal in future scaffold designs: integrins.
Integrins as a key component of tissue engineering
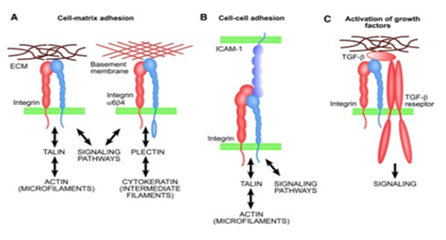
Integrins for cell adhesion: Integrins are a major family of surface receptors that facilitate cell adhesion.41 Integrins exist as heterodimers of noncovalently-linked α and β subunits, which bridge the cell-cell and cell-ECM interactions.41 Binding of integrins with specific ECM domains is of great importance in maintaining cell homeostasis, host defense and tissue maintenance.41 Figure 3 illustrates the basic functions of integrins.42 Aside from their mechanical role in attachment, integrins play a prominent role in cell signalling that provides information regarding the local environment, adhesive properties and surrounding matrix characteristics.41 Integrins are important in governing cell attachment to the biological scaffolds for tissue engineering, as a poor attachment will cause cells to grow abnormally and increase the chances of losing homeostasis.43 One study conducted on β1 integrins illustrated how IL-β1 is important in binding fibronectin matrices and can even facilitate adhesion when the matrix is damaged (Figure 4).44 IL-β1 is a cytokine that plays an important role in cell adhesion to damaged ECM by activating matrix metalloproteinases to reconstruct the matrix.44 However, the specific mechanisms behind cell attachment may also be dependent on the structure of the biological scaffold; 3D scaffolds that are highly porous may be able to entrap cells via a non-integrin based attachment.43 Nevertheless, proper cell attachment to tissue scaffolds continues to be a major consideration in tissue engineering design and research and integrins remain the central player in this regard.
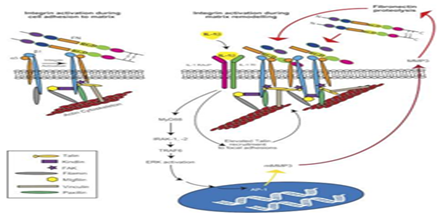
Integrin signalling: The binding of integrins to the ECM facilitates cell attachment via a signaling cascade that drives fibrillogenesis.45 Research on integrins is typically centered around uncovering the key players in this signaling cascade as well as the mechanisms that drive fibrillogenesis. A key example are transmembrane receptor tyrosine kinases known as discoidin domain receptors (DDR); while DDRs cannot form strong adhesions to collagen matrices, they have been shown to facilitate integrin-mediated cell adhesion of α1β1 and α2β1 via an unknown activation mechanism.45 Another key example is the role of α4β1 integrin, also known as VLA-4, in progenitor cell attachment.46 Furthermore, the innovation of lab-created integrin agonists, such as THI0019, has been shown to increase progenitor cell localization and adhesion via interaction with α4β1.46 THI0019 also showed cross-reaction and enhanced cell adhesion with the related integrins α4β7, α5β1 and αLβ2.46
Integrins in the immune response: The role of integrins in cell adhesion is not only important with regards to attachment of the proper cells to a desired ECM, but also in preventing the adhesion of immune cells that may occur during tissue rejection.47 The ephrin transmembrane receptor EphA2 has been shown to increase migration and adhesion of monocytes and macrophages via a cross interaction with α4β1 or αLβ2.47 Mechanisms such as those involved between Eph/ephrin signaling and integrin signaling is critical to keep in mind in tissue engineering designs, especially when taken in context with the previous discussion of integrin agonists.47 It is important to ensure that cell adhesion to the ECM is promoted for the desired seeded cells, while avoiding recruitment of immune cells .47
Integrins in tissue engineering: The importance of integrins is especially relevant when it comes to lab-created scaffolds. Using previous knowledge of integrin mechanisms, researchers have developed various models to predict how integrins will drive cell attachment.48 Furthermore, these models allow researchers to optimize tissue engineering methods to promote proper cell attachment and proliferation in lab-engineered scaffolds. A novel method known as surface-initiated assembly uses a fibronectin model to drive attachment between surface integrins and the ECM.48 The methodology is based on the mechanism of fibronectin dimers binding to integrin surface receptors, which in turn unfolds ECM proteins and drives assembly of insoluble matrix fibers.48 The SIA method uses thermal microprinting to exploit this fibronectin-integrin mechanism and create ECM nanofibers in the laboratory.48 On the cellular side of the fibronectin mechanism, it is known that αvβ3 and α5β1-integrins specifically play a role in cell adhesion.49 A recent study highlights the use of gold nanoparticle structured polyethylene glycol diacrylate micropillars (GPPP) as force sensors to elucidate the role of αvβ3 and α5β1-integrins in cell traction.49 Using pillar displacement as a metric, the researchers observed higher forces exerted with α5β1 mediated adhesion versus αvβ3 and a higher zyxin to actin ratio.49 This study holds great implications regarding protein recruitment at focal adhesion sites.49 These studies that illustrate and further uncover the importance of integrins in cell attachment to the ECM contribute greatly to future scaffold engineering designs.
3D bioprinting-the future of tissue engineering: Early tissue engineering attempts have been slowed down with many limitations.50 In order for in vitro organ engineering to serve as a solution for the transplantable organ shortage, it must be able to meet the demand while still remaining economical.50 Manufacturing of synthetic scaffolds can be costly and time-consuming.50 Although re-cellularized cadaver organ scaffolds have shown some promise, they also hold limitations.50 Previous methods are successful in providing scientists with the architecture needed to eventually develop a complete organ; however, they are lacking in the ability to obtain the specific cellular positioning necessary for proper organ development.50 This is especially true regarding development of the vascular system needed to ensure cell proliferation.50 The innovation of three-dimensional bioprinting has been able to surpass these limitations and pioneers like Organovo and Dr. Anthony Atala at the Wake Forest Institute for Regenerative Medicine have shown that 3D bioprinting is truly the future of tissue engineering.50
3D bioprinting methods: 3D bioprinting utilizes an additive manufacturing; this means that cells, growth factors, biomaterials and other functional components are added layer by layer in a controlled manner with the help of computerized machines.51 There are three main methods of bioprinting, each with their own working ideology: laser, inkjet and extrusion.52,53 Laser-assisted biological printers are based on the laser-induced forward transfer (LIFT) technique and provide the ability to allocate the material with micrometer resolution.54 Three things are required for laser bioprinting: a laser pulse source, a biological target ribbon and a substrate that acts as a receiver for the printed material.54 The ribbon is composed of a thin metal layer-usually gold or titanium-that can absorb the material.54 A cell suspension is placed on the ribbon’s surface and a laser pulse causes vaporization of the metal layer.54 This leads to the deposition of cells on the substrate in a pattern.54 With inkjet-based bioprinting, droplets of living cells are created and released accurately onto a surface, based on a computer-generated template.55 The beauty lies in its simplicity: a printer is used to print biological “ink” droplets onto biological “paper”.50 Bio-inks typically consist of cell suspensions and other biomaterials necessary to produce the organ scaffold and are loaded into an ink cartridge to be printed.50 Bio-paper is a colloquial term used to describe the substrate that the bio-ink is printed on.50 Advancements over the years, such as the introduction of computer-aided design and manufacturing (CAD/CAM) systems and multiple-head tissue/organ building systems (MtoBS), have added even more elegance to this concept.56 CAD/CAM software works by using computer input codes and algorithms to model an organ matrix in silico; this computer-generated model can then be relayed to an MtoBS for creation.56 Extrusion-based bioprinting is similar to inkjet printing in its use of these automated techniques.53 Extrusion-based printing utilizes a three-axis automated robotic system to dispense fluid matrix components in cylindrical filaments, rather than droplets.53 Biomaterial is dispensed with the aid of a robotic pressure-aided system that allows for layer-by-layer matrix creation.53 As 2D layers form and stack on top each other, they cross-link to eventually form a 3D structure.50
3D printing a whole organ: 3D bioprinting holds the most promise in solving the problem of an organ shortage by allowing scientists to create their own organ supply, essentially from scratch 50 however, it is still in its early stages in terms of regenerative medicine.50 While scientists have been able to successfully create and transplant simple structures, typically of one tissue type, they have yet to do the same with more complex organs.50 Limitations are met when it comes to complex organs that consist of multiple cell types and have very intricate microstructures.56 While the success of a 3D-printed bladder is no small feat, it is relatively simple because it is a hollow organ.56 That is not the case with many of the body’s vital organs.56 Scientists needed to find a way to address the difficulty in printing the intricate network of cavities, tubules and vasculature that fill these vital organs and are necessary for proper function.56 The introduction of “fugitive inks” has allowed scientists to begin to address these difficulties.56 Fugitive inks are bio-inks that are made of a material that can be later dissolved, such as gelatin or pluronics, thus leaving behind a hollow cavity.56 CAD/CAM algorithms are used to model and design the organ to be printed.56 An MtoBS system is then used to systematically add the various constituents necessary to build the complex microstructure of the organ; one of these constituents is the fugitive ink.56 The fugitive ink is then dissolved and replaced by the appropriate cell suspension for the organ in question.56 These methods have already been proven in laboratory studies and are expected to be used in complex organ development in the near future.56 With more research and continuing advancements, 3D bioprinting may one day become the default approach to organ transplantation. There is still quite a bit of research and optimization to create a viable printed organ. Moreover, studies still need to be performed on any potential long-term immune response to these 3D printed organs. Nevertheless, 3D bioprinting represents the future of regenerative medicine.
Chronic kidney disease-the reality of regenerative medicine
As we review these various topics in tissue engineering, it is easy to get lost in the pomp and circumstance of it all. Yet these innovations hold little value without a real-world model to add context. 26 million Americans have chronic kidney disease (CKD), with millions more at risk.5 The difficulty in addressing CKD is that there is no true “cure”.5 CKD is often caused by diabetes or high blood pressure and so treatment involves controlling these underlying ailments to prevent complete renal failure.5 Renal failure is typically addressed with hemodialysis; however, if the individual progresses to late-stage CKD, then the only option is a kidney transplant.5 The National Kidney Foundation estimates that thirteen people die every day waiting for an available kidney.5 Of the individuals who undergo kidney transplantation, there still lies a concern of organ rejection.57 Recent studies show that using small molecule drugs to inhibit the enzymatic activity of spleen tyrosine kinase can interrupt the signal cascade and diminish the immune response to the donor kidney.57 Yet this does nothing to resolve the organ shortage for the many who are in need.57 The advent of organ decellularization and re-seeding introduce a potential solution to this issue. Studies in rats showed that decellularized organ scaffolds that are re-seeded with epithelial progenitor cells can be applied to damaged kidneys and lead to regeneration.57 Molecular cues from growth factors are added to induce differentiation into various kidney cell types.57 This combination of growing stem cells on decellularized scaffolds can be up scaled to engineering of a whole organ.58,59 Typically, the decellularized scaffolds come from cadavers or other animals; the scaffolds are re-seeded with stem cells and grown in a bioreactor.58,59 The limitations attributed to this method come with the cost of time and resources, as well as the ability to ensure that the cells grow and differentiate appropriately.58,59 The bioreactors create an environment for cell proliferation that is supposed to mimic the human body, however scientists have limited control over these processes.58,59 Aside from these limitations, research is still necessary to investigate the true compatibility of the extracellular matrix between other animals and humans.60 Also, scaffolds coming from cadavers may have been affected by other disease states.60 Even if these issues are resolved, it still keeps patients on a waiting list until the organ becomes available.60 The only true solution would be one in which waiting individuals can receive the kidney they need as soon as it becomes necessary, not available.60 3D bioprinting provides this solution by allowing scientists to create the kidneys as they are needed.60
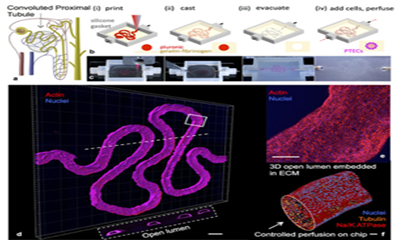
Yet the field of regenerative medicine is not quite at the point of being able to mass produce functional kidneys.60 The difficulty in 3D printing an entire kidney can be attributed to the organ’s complexity.60 Dr. Atala demonstrated that it is possible to create what appears to be the main structure of the kidney as well as miniature organ models.60 In order to create a kidney that is fully functional, scientists need to be able to create the micro-architecture of nephrons-the glomerulae and its vasculature.60 It was mentioned earlier how scientists plan to do this using fugitive inks.60 A model for the kidney’s proximal tubules has now been created using these techniques.61 Researchers at Harvard’s Wyss Institute. 3D-printed a section of kidney microtubule as a 3D “organ-on-chip” model by using pluronic set in a gelatin/fibrinogen matrix and then later replacing the pluronic fugitive ink with kidney microtubule cells (Figure 5).61 Analysis showed that this 3D kidney model exhibits cyclosporine toxicity and albumin uptake as they would in the human body (Figure 6).61 This 3D model represents an improvement over 2D organ-on-chip models and a large step closer to developing a fully-functional kidney.61 What once seemed like science fiction is now getting closer and closer to reality. These innovations have brought the field of regenerative medicine within reach of fabricating whole, fully functional organs. For the many who are suffering from CKD, waiting for the phone call to receive their new kidney, this research provides a welcome light at the end of the tunnel.

None.
The author declares no conflict of interest.

©2017 Lobos, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.