Journal of
eISSN: 2572-8466


Research Article Volume 1 Issue 3
Able Corporation, Japan
Correspondence: Makoto Shoda, Able Corporation, 7-9 Nishigoken-cho, Shinjuku-ku, Tokyo, 216-0812 Japan, Tel 81-3-260- 0485, Fax 81-3-260-0485
Received: September 19, 2016 | Published: December 30, 2016
Citation: Shoda M, Ishikawa Y. Removal of high-strength of ammonium and phenol from coking wastewater by alcaligenes faecalis No.4. J Appl Biotechnol Bioeng. 2016;1(3):82-85. DOI: 10.15406/jabb.2016.01.00014
Alcaligenes faecalis No.4, which is capable of heterotrophic nitrification and aerobic denitrification was used to treat coking wastewater (CW) to remove high-strength of ammonium and phenol. First, the phenol-degrading ability of No.4 was verified and a synthetic medium containing lactate and phenol as carbon sources was subsequently applied to the No.4 culture. Both carbon materials were entirely utilized for the complete consumption of ammonium. Next a coking wastewater containing 400mg/l of phenol and 400mg/l of ammonium-nitrogen was treated by the No.4 culture. Both compounds were completely removed through the addition of 4g/l of lactate. The removal rate of ammonium was 1.1kg-N/m3/day which is several hundred-fold higher than that in conventional treatment system.
Keywords: heterotrophic nitrification, aerobic denitrification, high-strength ammonium, alcaligenes faecalis, phenol-containing wastewater
DO, dissolved oxygen; CW, coking wastewater; EDTA, (2,2',2'',2'''-(Ethane-1,2-diyldinitrilo)tetra acetic acid
Coking wastewater (CW) originates from the process of destructive distillation of coal at high temperatures in the absence of air. Phenols are the major constituents of the coking wastewater and can seriously inhibit various biological reactions, especially the nitrification reaction. Conventional biological treatment is difficult mainly due to refractory substances. When high-strength ammonium is involved in CW, COD in the wastewater is not sufficient to complete the removal of ammonium.1-5 Recently, many bacteria have been known to be capable of heterotrophic nitrification and aerobic denitrification.6-14 The use of these bacteria provides advantages over the conventional nitrogen removal process of aerobic nitrification and an anaerobic denitrification because ammonium removal is achieved in one reactor using one type of bacteria under aerobic conditions. These ammonium removal rates are higher than those in conventional ammonium removal process, primarily because of short hydraulic retention time. In a previous study,15 we demonstrated that A. faecalis No.4 (No.4) has the ability to carry out the following heterotrophic nitrification and aerobic denitrification
NH4+ → NH2OH → N2O → N2
Approximately 40% and 60% of ammonium were converted to N2 gas and cell mass respectively and only a small amount of ammonium was converted to NO2- and NO3-. No.4 removed more than 90% of high-strength ammonium and COD from crude piggery wastewater without diluting it.16 No.4 also exhibited an ammonium removal rate of 3kg- NH4-N/m3/day in the treatment of anaerobically digested sludge from a municipal wastewater plant.17 A wastewater from a chemical company, which contained a high concentration of ammonium (5,000mg-NH4-N/L) and a small amount of BOD was treated by No.4 and the average ammonium removal rate was 1.1kg-NH4-N/m3/day.18 These removal rates by No.4 were several hundred-fold higher than that in conventional treatment method.19,20 In this study, No.4 was applied to a coking wastewater supplied by a chemical company to assess the effects of biological treatment of high-strength ammonium and phenol using a 1-liter jar fermenter.
Strain used
Characteristics of No.4 have been previously described in details.11 Cultured cells of No.4 were mixed with a 50% glycerol solution in vials and stored at -84°C. For each pre-culture one vial was used as the No.4 inoculum.
Medium used
A synthetic medium containing 14g/l of K2HPO4, 6g/l of KH2PO4, 12.5g/l of sodium lactate, 2g/l of (NH4)2SO4 and 0.2g/l of MgSO4・7H2O in 2ml of trace mineral solution was used for the pre-culture of No.4. The trace mineral solution contained the following components (g per liter): 57.1 EDTA (2,2',2'',2'''- (ethane-1,2-diyldinitrilo)tetra acetic acid)・2Na, 3.9 ZnSO4・7H2O, 7 CaCl2・2H2O, 5.1 MnCl2・4H2O, 5.0 FeSO4・7H2O,1.1 (NH4)6Mo7O24・4H2O, 1.6 CuSO4・5H2O and 1.6 CoCl2・6H2O. No.4 utilizes fatty acids as a carbon source and in a previous study citrate was used. When lactate and citrate were compared as carbon source the ammonium utilization rate and the growth rate of No.4 were significantly higher than those when lactate was used. Therefore, lactate was used in the following experiments. The synthetic medium was sterilized for the use of pre-culture but in batch treatment culture, no sterilization was conducted. Phenol was added without sterilization.
Wastewater used
The coking wastewater (CW) was supplied by a Japanese chemical company. The primary characteristics of the CW are as follows: pH 8.5, total COD concentration of 5,200mg/l, ammonium-nitrogen concentration of 800mg/l and phenol concentration of 820mg/l. In each experiment the pH of the original CW was adjusted to approximately 7.5 by 5NH2SO4 and the pH-adjusted CW was diluted arbitrarily.
Reactors used
A small-scale jar fermenter (total volume of 1 liter, working volume of 300 ml; BMJ-01PI, Able Corp., Tokyo, Japan) was used. The dissolved oxygen (DO) concentrations and pH values were monitored with a DO sensor (SDOC-12F, Able Corp., Tokyo, Japan) and a pH sensor (Easyferm plus 225, Hamilton Bonaduz AG, Bonaduz, Switzerland) inserted into the fermenter. The temperature was maintained at 30°C. The agitation speed was controlled at 600 rpm with a constant air supply rate of 30ml/min.
Experimental procedure
The synthetic media were prepared in the pre-culture of No.4 containing lactate (10g/l) and phenol (0.2g/l) or lactate only. No.4 was cultivated in the two media for 3 days, when lactate and phenol were consumed completely. Next, the pre-cultures were centrifuged at 10,000 rpm for 10 min and the collected cells of No.4 were washed with 0.1M phosphate buffer two times. The cells were inoculated into the synthetic medium which was devoid of lactate and contained only phenol as a carbon source. In CW treatment, pre-cultured No.4 cells were introduced into different dilution CW and the growth of No.4 was confirmed at 50% dilution in shaking flasks. The diluted CW was added with lactate and No.4 culture in jar fermenter and ammonium removal test was conducted.
Analytical method
Ammonium concentration was determined using an ammonium sensor (SNH-10, Able Corp., Tokyo). For phenol concentration determination, the chemical analysis kit for phenol (LR-PNL, Kyoritsu Chemical-Check Lab., Corp., Tokyo, Japan) was used. The initial and final values of TOC in the prepared solution were determined at Kuritasu Analyzing CO., Ltd., (Tukuba, Japan). For determining the cell number of No.4, the sampled culture was diluted and plated on the synthetic agar plates which contained the synthetic medium and 1.5% agar and then the plates were incubated at 30°C for 2 days. Since it was previously confirmed that No.4 grew on the plates significantly faster than other cells indigenous to the CW and exhibited their characteristic morphological features the colonies appeared on the plates after 2 days were counted as No.4. The cell concentration was expressed as cells/ml. The crude coking wastewater was streaked on the LB medium and the synthetic agar medium and no colonies appeared after 3 days of incubation. Therefore, indigenous microorganisms in the crude coke-production wastewater were negligible in number. The air was supplied to the CW sample containing lactate in the jar fermenter for 4 days and neither the removal of lactate nor ammonium was observed and thus the air-borne microorganisms from outside reactor were also negligible in the treatment.
Availability of phenol by No.4
Figure 1 shows the change in concentrations of ammonium and phenol in the synthetic medium devoid of lactate when phenol was not added in the pre-culture. Figure 2 shows the change in concentrations of ammonium and phenol when phenol was added in the pre-culture. Comparison of the two figures, phenol degradation rate and ammonium removal rate were 38 (mg/l/h) and 5.3 (mg/l/h) for Figure 2 and 31 and 4.1 for Figure 1, respectively. As shown in Figure 2, 18 h were needed for the complete removal of phenol while 22h, as shown in Figure 1. This finding indicates that phenol was utilized by No.4 and the induction of phenol-degradation ability by phenol in the pre-culture was associated with the enhanced removal rates and shorter time to exhaust phenol. At the moment when phenol was exhausted, DO rapidly increased, indicating that the consumption of phenol and DO change were well-correlated. When the initial phenol concentration was 600-700mg/l, this includes 459-535mg/l of carbon. If the C/N ratio of cell synthesis was 10, consumption of 600 to 700mg/l of phenol corresponded with the consumption of only approximately 50 to 60mg/l of ammonium-nitrogen. The consumption of ammonium-nitrogen in Figure 1 & 2 well reflected the ratio. The ammonium removal rates using phenol as a carbon source in the two experiments were 0.098-0.12 kg-N/m3/day, which is approximately one-tenth of the rate when fatty acids were used as a carbon source. However, these data were approximately 10-fold higher than the rate in conventional nitrification-denitrification method. This finding suggests that for complete removal of ammonium in CW, addition of available carbon for No.4 is needed.
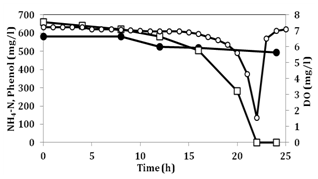
Figure 1 Removal of ammonium and phenol in synthetic medium, where phenol is a sole carbon source by No.4 when lactate was used as a carbon source in pre-culture.
Symbols: (●): NH4-N concentration, (□): phenol concentration and (○): Dissolved oxygen(DO) concentration.
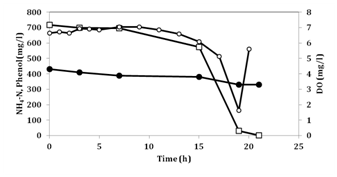
Figure 2 Removal of ammonium and phenol in synthetic medium, where phenol is a sole carbon source by No.4 when lactate and phenol were used as a carbon source in pre-culture.
Symbols: (●): NH4-N concentration, (□): phenol concentration and (○): Dissolved oxygen(DO) concentration.
Ammonium removal when lactate and phenol were mixed in synthetic medium
The No.4 cells prepared in the pre-culture containing phenol were introduced into the synthetic medium containing lactate and phenol. The change in concentrations of ammonium, phenol and lactate is shown in Figure 3. The initial ammonium-nitrogen concentration, phenol concentration and lactate concentration were 733mg/l, 636mg/l and 4.8g/l, respectively. In this case, higher amount of ammonium was added to observe the clear change of phenol and lactate. At the exhaustion of lactate, approximately 500mg/l of ammonium-nitrogen was utilized and after the decline in lactate concentration, phenol degradation started and 50 mg/l of ammonium-nitrogen was consumed. This shows that lactate consumption begins first and subsequently the consumption of phenol takes place. The initial cell number of No.4, 2x108cells/ml, increased to 8x 109cells/ml.
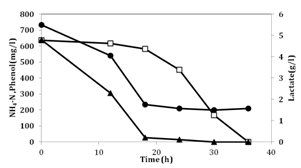
Figure 3 Removal of ammonium, phenol and lactate in the synthetic medium.
Symbols: (●): NH4-N concentration, (□): Phenol concentration and (▲): Lactate concentration.
Coking wastewater treatment by No.4
The No.4 culture was directly mixed with crude coking wastewater with fortified lactate in a jar fermenter but removal rates of ammonium and lactate were significantly decreased. The ammonium removal rates and phenol consumption rates were 0.014kg-N/m3/day and 0.0035kg/m3/day, respectively. Presumably, toxic substances in coking wastewater inhibited the activity of No.4. This finding indicates that carbons in the crude wastewater were not degradable by No.4 and addition of organic acids may be needed for efficient treatment of ammonium and phenol. When CW was diluted the normal growth of No.4 was observed at 50% dilution. Next, 50% of dilution CW wastewater was mixed with No.4 pre-culture and 4g/l of lactate. The result is shown in Figure 4. The initial ammonium-nitrogen concentration, phenol concentration and lactate concentration were 420mg/l, 380mg/l and 4g/l, respectively. The ammonium removal rate was 1.8kg-N/m3/day and phenol removal rate was 0.7kg/m3/day. The ammonium removal rate was 50-60 times higher than the previous results that treated phenol.4,5 Phenol removal rate 0.7kg/m3/day was two times larger than that in the previous report.5
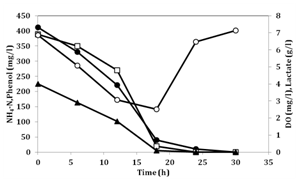
Figure 4 Removal of ammonium, phenol and lactate in coking wastewater.
Symbols: (●): NH4-N concentration, (□): Phenol concentration, (▲): Lactate concentration and (○): Dissolved oxygen(DO) concentration.
COD in the initial wastewater containing lactate was 12,000mg/l and after the treatment this value decreased to 2,830mg/l. The COD of 50% diluted CW was 2,130mg/l. Thus, this ammonium treatment was primarily undertaken by No.4 by consumption of added lactate and indigenous phenol. These data indicate that addition of lactate stimulated the activity of No.4 and lactate, ammonium and phenol were removed simultaneously. When lactate concentration was significantly lower, the consumption of phenol was initiated. As coking wastewater contained some other carbon substances not available for No.4, further treatment may be needed for complete treatment of COD after this system. As we showed in previous papers, No.4 is useful for ammonium removal under high saline condition17,18 and the cells of No.4 have ability to suppress the plant pathogens21 and to reduce methane production from rumen.22 This suggests that the reutilization of the excess cells of No.4 after treatment is possible and this reduces the problem of handling of the excess sludge produced after wastewater treatment.
Since A. faecalis No.4 exhibits phenol degradation activity the effects of its treatment on phenol-containing wastewater were determined under high-strength-ammonium condition. In pure culture, No.4 showed degradation ability for 700mg/l phenol the concentration that was inhibitory to nitrifying bacteria. In the synthetic medium containing phenol, lactate and ammonium, all three components could be utilized when COD to nitrogen ratio is properly arranged. In diluted coking wastewater, 400mg/l of ammonium-nitrogen and 380mg/l of phenol were removed and the ammonium removal rate was 50-fold larger than that reported in previous papers dealing with phenol treatment and 100-fold higher than that observed in the conventional ammonium treatment system.
None.
The author declares no conflict of interest.

©2016 Shoda, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.