International Journal of
eISSN: 2573-2889


Research Article Volume 4 Issue 4
1Department of Microbiology, Faculty of Science, Adekunle Ajasin University, Nigeria
2Department of Life Sciences, University of Modena and Reggio Emilia, Italy
Correspondence: Oludare Temitope Osuntokun, Department of Microbiology, Faculty of Science, Adekunle Ajasin University, Akungba-Akoko, Ondo state, Nigeria, Tel 08063813635
Received: August 08, 2019 | Published: August 26, 2019
Citation: Osuntokun OT, Cristina GM. Bio isolation, chemical purification, identification, antimicrobial and synergistic efficacy of extracted essential oils from stem bark extract of Spondias mombin(Linn). Int J Mol Biol Open Access. 2019;4(4):135?143. DOI: 10.15406/ijmboa.2019.04.00110
The purpose of this research work is to isolate, purify, chemical identification, antimicrobial and Synegistic efficacy of extracted essential oils from ethyl acetate extract of Spondias mombin. The ethyl acetate stem bark extract of Spondias mombin were air-dried, chopped into smaller pieces and cold extracted exhaustively with ethylacetate. The crude extract was partitioned using various solvents and the dichloromethane fraction was concentrated and fractionated using column chromatography packed with Si-gel and Sephadex-LH and eluted with appropriate solvent systems accordingly. In order to obtain pure extracts, partially purified fractions were further purified. The structures of the isolated compounds were determined by using data obtained from GC-MS spectrum. The compound isolated includeAspidofractinine-3-methanol, Phthalic acid, 2-ethylhexyl tetradecyl ester, Phthalic acid, di (2-propylpentyl) ester), (9-(2', 2’-Dimethylpropanoilhydrazono)-3,6-dichloro-2,7-bis-[2-(diethylamino)-ethoxy]fluorine) and Tere phthalic acid, dodecyl 2-ethylhexyl ester.These compounds have individual and synergistic activity against Gram negative (E.coli), Gram-positive (Bacillus subtilis) and (Aspergillusflavus) at 10, 5, 2.5 and 1.25g/mL.
Keywords: spondias mombin, antimicrobial, essential oils, gc-ms
The search for drugs from natural sources has been on the increase in ethno-medicinal research1 and plant kingdom has served as an inexhaustible source of useful drugs, foods, additives, flavouring agents, lubricants, colouring agents and gums from time immemorial.2 Medicinal plants have been used since time immemorial in virtually all societies as source of medicine to combat various ailments including infectious diseases. The therapeutic power of herbs has been recognized since creation of the universe and botanic medicine is one of the oldest practiced professions by mankind.3 Medicinal plants contain large varieties of chemical substances with important therapeutic properties that can be utilised in the treatment of human diseases.4,5 The antibacterial, antimicrobial, antiviral and antifungal potentials of the plant have been reported.6–9 The plant was recommended for use by pregnant women after five months of pregnancy10 while the mineral analysis of the plant has also been documented. 11,12 The abortifacient activity of an aqueous extract of Spondias mombin was reported by Offiah et al. 13 The plant has been shown to have a wide range of phyto constituents such as tannins, saponins and anthraquinone glycosides. 7 The plant has been reported to be used in the treatment of many disease conditions in the Eastern part of Nigeria by the natives. The associated link between the composition of this world-wide cultivated plant and the reported medicinal and economic values prompted this study aimed at evaluating the phytochemical and antioxidant properties as well as the microbial inhibitory effects of the leaf and stem bark extracts of the plant.
Collection of plant materials
The stem bark of Spondiasmombin was collected from a local farm at Owo (710’59.998N and 534’59.988E), Ondo State, Nigeria at WAT UTC+1 time zone on 26th and 27th of February, 2016. Fresh and healthy plants were also collected during its fruiting season, between April and July 2017 from the same geographical locations.
Authentication of plant samples
The plants were authenticated at the Department of Plant Science and Biotechnology, AdekunleAjasin University, Akungba- Akoko, Ondo state, Nigeria.
Extraction and isolation from Spondiasmombin plant
The stem-bark of Spondiasmombin plant were harvested and air-dried. The dried bark was milled into powdered form using manual grinder. Powdered plant material (1kg) each of the different plant parts was extracted with 3L of 70% (v/v) ethanol, ethyl acetate and distilled water for 72 hours at room temperature. The extraction process was repeated four times until the extract became clear. The filtrates were combined and concentrated in vacuo using rotatory evaporator at 35°C. The fraction was labeled SMSBEA and kept in tightly stoppered bottles in a refrigerator at -4oC for further analysis.
Isolation and purification of extracted essential oil (eo) from stem bark of spondiasmombin (linn)
The crude extract (20g) of S. mombin was adsorbed onto silica and successively eluted with solvents and the dichloromethane fraction was obtained, concentrated and dried. From the dichloromethane fraction, 5.8g was adsorbed unto silica gel (200–400mesh). The column was degased with n-hexane and thin layer chromatography guided gradient elution was carried out using n-hexane, n-hexane-dichloromethane (60:30) and n-hexane-dichloromethane (65:35) respectively. The two later elutions were bulked and labelled Fraction 2B weighing 3.8g. Fraction 2B was subjected to column chromatography, degased and was eluted with n-hexane-dichloromethane in the following respective proportions, 65:35, 60:40, 55:45, 50:50, 45:55, 40:60 and 35:65 respectively. The elution from 50:50 and 40:60 were bulked and concentrated in-vacuo. The elution from 30:70 was also concentrated as well. The concentrates were placed on Sephadex LH-20 and eluted with dichloromethane-methanol (70:30) giving five pure extracts labelled A3, B3, C3, E3, and F3. The compounds were then analysed using FT-IR Spectrophotometer (Perkin Elmer) over the frequency range from 4000-400 cm-1. UV-VIS and FT-IR analysis was performed at National Institute of Pharmaceutical Education and Research (NIPER), Mohali (India), The GC-MS analysis of the compounds was performed using a Perkin Elmer GC Claurus 500 system and gas chromatograph interfaced to a Mass Spectrometer (GC-MS) equipped with Elite-1 fused silica capillary column (30 m×1µl was Mdf. Composed of 100% Dimethyl poly siloxane)
Antibacterial and synergistic assay of extracted essential oil (eo) fromstem bark of Spondiasmombin (Linn)
Antibacterial and antifungal screening of the extract: All the test bacteria, were sub-cultured onto sterile Mueller Hinton agar plates, and incubated at 37°C for 18-24 hours. Five distinct colonies for each organism were inoculated onto sterile Mueller Hinton broth and incubated for 3- 4hours. All inocula were standardized accordingly to match the 0.5 McFarland standards, and this standard was used for all susceptibility tests. All the extracts were reconstituted accordingly into the following concentrations; 10, 5, 2.5, 1.25mg/ml, The susceptibility testing was investigated by the agar well diffusion method. A 0.1ml of 1: 10,000 dilution (equivalent to 106cfu/ml) of fresh overnight culture of the clinical isolates grown in Mueller Hinton agar and Sabouraud dextrose agar was seeded into 40ml of Mueller Hinton agar, and properly mixed in universal bottles. The mixture was aseptically poured into sterile Petri dishes and allowed to set. Using a sterile Cork borer of 6mm diameter, equidistant wells were made in the agar. Drops of the re suspended, (2ml per well) extracts with concentrations between 10, 5, 2.5, 1.25mg/mL, were introduced into the wells till it was filled. The plates could stand on the bench for an hour, to allow pre-diffusion of the extracts before incubation at 37°C for 24 hours for the bacterial isolates and 24°C for 48 hours for the fungal isolates. The zones of inhibition were measured to the nearest millimeter (mm) using a standard transparent meter rule. All experiments were performed in duplicates.14–18
Gas chromatography and mass spectroscopy gc/ms method of analysis
GC-MS technique was used in this study to identify the components present in the extract. GC-MS technique was carried out at Indian Institute of Crop Processing Technology (IICPT) Thanjavur, Tamilnadu. GC-MS analysis of this extract was performed using a Perkin Elmer GC Claurus 500 system and gas chromatograph interfaced to a Mass Spectrometer (GC-MS) equipped with Elite-1 fused silica capillary column (30mx1µl was Mdf. Composed of 100% Dimethyl poly siloxane).19 For GC-MS detection, an electron ionization energy system with ionization energy of 70eV was used. Helium gas (99.999%) was used as the carrier gas at a constant flow rate of 1ml/min. and an injection volume of 2µl was employed (Split ratio of 10:1). Injector temperature was 250ºC. The oven temperature was programmed from 110ºC (isothermal for 2min.), with an increase of 10ºC/min to 200ºC, then 5ºC /min. to 280ºC, ending with a 9min. isothermal at 280ºC. Mass spectra were taken at 70eV; a scan interval of 0.5 seconds and fragments from 45 to 450 Da. Total GC running time was 36 min. The relative percentage amount of each component was calculated by comparing its average peak area to the total areas. Software adopted to handle mass spectra and chromatograms was a Turbo mass Ver5.2.0. Compound identification was obtained by comparing the retention times with those of authentic compounds and the spectral data obtained from library data of the corresponding compounds. The given sample was extracted with ethyl acetate and analyzed in GC-MS for different component (Figure 1).
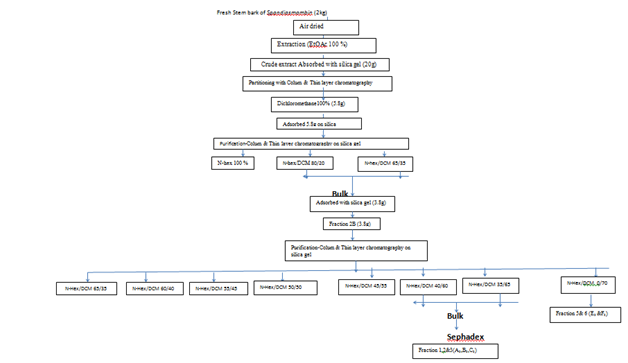
Figure 1 Isolation and purification of extracted essential oil (eo) from stem bark of Spondiasmombin (Linn).
Key: A3 - C20H26N2O- Aspido fractinine-3-methanol,(2.alpha.,3.beta.,5.alpha) B3- C30H50O4- Phthalic acid, 2-ethylhexyl tetradecyl ester C3- C24H38O4- Phthalic acid, di(2-propyl pentyl) ester E3- C30H42Cl2N4O3- 9-(2',2'-Dimethyl propanoilhydrazono)-3,6-dichlo ro-2,7-bis-[2-(diethylamino)-ethoxy ]fluoreneF3- C28H46O4 - Terephthalic acid, dodecyl 2-ethylhexyl ester
Structural profiling of pure compound of stem bark extracts of Spondiasmonbin using gas chromatography and mass spectrometric(gc/ms) method of identification
Table 1 Shows the Chromatogram A3 of Gas chromatography and mass spectrometry (GCMS) of compound A3, The pure extract contained arrays of compounds, whichare listed as follows; Undecane(C11H24), Dodecane(C12H26), 3,9-dimethyl-(C13H28), 5-Ethylundecane(C13H28), 2-Naphthalenol, 1,2-dihydro-, acetate(C12H12O2), Azulene(C10H8), Naphthalene(C10H8), N-Methyl-9-aza-tricyclo[6.2.2.0(2,7)]dodec-2,4,6,11-tetraene-10-one(C12H11NO), 1H-Indene, 1-methylene (C10H8), Tridecane(C11H24), Decane,2,9-dimethyl(C12H26), 3,6-Dimethylundecane (C13H28), Octan e,2,3,7-trimethyl- (C11H24), 3-Ethyl-5-methylheptane(C10H22), 2,7,10-Trimethyldodecane (C15H32), Nonane, 3-Methyl-5-pro pylon nane (C13H28), Hexadecane, 2,6,10,14-tetramethyl-, Phytan(C20H42), 3-Methyl-5-propylnonane(C13H28), 2,6,10-Trimethyl dodecane, Farnesane (C15H32), Hexadecane, n-Cetane(C16H34), 1,2-Benzenedicarboxylic acid, butyl 2-methylpropyl ester, (Phthalic acid, butyl isobutyl ester)(C16H22O4), 2-Octyl benzoate(1-Methylheptyl benzoate)(C15H22O2), Benzoic acid, 2-ethylhexyl ester (C15H22O2), Oxalic acid, allylhexadecyl ester(C21H38O4), 2-Piperidinone, N-[4-bromo-n-butyl]-(1-(4-Bromobutyl)-2-piperidinone)(C9H16BrNO), 1H-Indene, 1-methylene-(1-Methylene-1H-indene)(C10H8), 1,2-Benzenedicarboxylic acid, butyl 2-methylpropyl ester (C16H22O4), Dibutyl phthalate (1,2-Benzenedicarboxylic acid, dibutyl ester)(C16H22O4), Eicosane, 10-methyl-10-Methylicosane (C21H44).
|
Name |
Molecular weight |
Structural formula |
Retindex |
|
ndecane |
156 |
C11H24 |
1115 |
|
Tridecane |
184 |
C13H28 |
1313 |
|
Adakane 12 |
170 |
C12H26CH3(CH2)10CH3 |
1214 |
|
Dimethylundecane |
184 |
C13H28 |
1185 |
|
5-Ethylundecane |
184 |
C13H28 |
1249 |
|
2-Naphthalenol, 1,2-dihydro-, acetate |
188 |
C12H12O2 |
1476 |
|
Aspidofractinine-3-methanol, (2.alpha.,3.beta.,5.alpha.)- |
310 |
C20H26N2O |
2510 |
Table 1 Chromatogram of aspido fractinine-3 methanol,(2.alpha,3.beta,5 alpha, using gas chromatography and mass spectroscopy gc/ms method of analysis (Mass Peaks: 395, 400)(A3)
Table 2 Shows the Chromatogram B3 of Gas chromatography and mass spectrometry (GCMS) of compound B3. The pure extract contained arrays of compounds, which listed as follows; Dodecane (Adakane 12)(C12H26), Undecane(Hendecane)(C11H24), Decane (2,9,Dimethyldecane)(C12H26), Octane, 2,3,7-trimethyl(C11H24), Tridecane, 7-methyl(Methyltridecane)(C14H30), Heptane(3-Ethyl-5-methylheptane)(C10H22), Nonane, (3-Methyl-5-propylnonane)( C13H28), Diisooctylphthalate (Bis(6-methylheptyl) phthalate, 1,2-Benzenedicarboxylic acid, 1,2-Benzenedicarboxylic acid(C24H38O4), 1,2-Benzenedicarboxylic acid, diheptyl ester(C22H34O4), Phthalic acid, 5-methylhex-2-yl heptyleste (C22H34O4), 8-Hexadecenal, 14-methyl-, (Z)-(14-Methyl-8-hexadecenal, (8Z)-14-Methyl-8-hexadecenal (C17H32O), 7-Hexadecenal, (Z)(Z)-7-Hexadecenal, Z-7-Hexadecenal, (7Z)-7-Hexadecenal(C16H30O), cis-9-Hexadecenal(C16H30O), Heptadecane, 2,6,10,15-tetramethyl-2,6,10,15-Tetramethylheptadecane(C21H44), 9-Octadecenal, (Z)- (Olealdehyde)(C18H34O), 9-Octadecenoic acid, 1,2,3-propanetriyl ester, (E,E,E),(2,3-Bis[(9E)-9-octadecenoyloxy]propyl (9E)-9-octadecenoate)(C57H104O6), n-Propyl 9,12-octadecadienoate, n-Propyl linoleate (C21H38O2), 9,12-Octadecadienoic acid (Z,Z)-, 2-hydroxy-1-(hydro xymethyl) ethyl ester(Linolein, 2-mono- .beta.-Monolinolein)(C21H38O4), Butyl 9,12-octadecadienoate (C22H40O2), Bis(tridecyl) phthalate(1,2-Benzenedicarboxylic acid, ditridecyl ester (C34H58O4), Ethyl 13-methyl-tetradecanoate(C17H34O2), Eicosanoic acid, ethyl ester, Ethyl icosanoate, Ethyl arachidate (C22H44O2).
|
Name |
Molecular weight |
Structural formula |
Retindex |
|
Dodecane |
170 |
C12H26 |
1214 |
|
Tridecane |
184 |
C13H28 |
1313 |
|
Hendecane |
156 |
C11H24 |
1115 |
|
11Methyldodecane |
184 |
C13H28 |
1249 |
|
Decane, 2,9-dimethyl- |
170 |
C12H26 |
86 |
|
Octane, 2,3,7-Trimethyloctane |
156 |
C11H24 |
922 |
|
Tridecane, 7-methyl- 7-Methyltridecane |
198 |
C14H30 |
349 |
|
3-Ethyl-5-methylheptane |
142 |
C10H22 |
887 |
|
Phthalic acid, 2-ethylhexyl tetradecyl ester |
474 |
C30H50O4 |
3364 |
Table 2 Chromatogram of Phthalic acid, 2-ethylhexyl tetradecylester,using gas chromatography and mass spectroscopy gcmsmethod of analysis (Masspeaks:395,400) B3
Table 3 Shows the Chromatogram C3 of Gas chromatography and mass spectrometry (GCMS) of compound C3. The pure extract contains arrays of compounds, which listed as follows; 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester(C16H22O4), Dibutyl phthalate(n-Butyl phthalate $$ Butyl phthalate )(C16H22O4 ), Phthalic acid, hex-3-yl isobutyl ester(C18H26O4), 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester(Diisobutyl phthalate)(C16H22O4), 2-Benzenedicar boxylic acid, butyl 2-ethylhexyl ester (C20H30O4), 1,2-Benzenedicarboxylic acid, diheptyl ester( Diheptyl phthalate)(C22H34O4), Phthalic acid, 5-methylhex-2-yl heptyl ester(C22H34O4), Phthalic acid, 4,4-dimethylpent-2-yl heptyl ester(C22H34O4), Diisooctyl phthalate (1,2-Benzenedicarboxylic acid, bis(6-methylheptyl) ester)(C24H38O4), Bis(2-ethylhexyl) phthalate, Phthalic acid, bis(2-ethylhexyl) ester Bisoflex 81 (C24H38O4), Phthalic acid, di(2-propylpentyl) ester(C24H38O4), Phthalic acid, di(6-methylhept-2-yl) ester(C24H38O4), Phthalic acid, 2-ethylhexyl tetradecyl ester (C30H50O4), Phthalic acid, octyl 2-propylpentyl ester (C24H38O4),Phthalic acid, 2-ethylhexyl undecyl ester(C27H44O4), Phthalic acid, decyl 2-ethylhexyl ester(C26H42O4), Diisooctylphthalate, Bis(6-methylheptyl) phthalate(C24H38O4), Bis(2-ethylhexyl) phthalate (Phthalic acid, bis(2-ethylhexyl) ester, Bis(2-ethylhexyl) 1,2-benzenedicarboxylate (C24H38O4), Bis(tridecyl) phthalate(1,2-Benzenedicarboxylic acid, ditridecyl ester)( C34H58O4), Phthalic acid, octyl oct-3-yl ester(C24H38O4), Phthalic acid, dodecyl octyl ester(C28H46O4), Phthalic acid, heptyloctyl ester(1-Heptyl 2-octyl phthalate)(C23H36O4).
|
Name |
Molecular weight |
Structural formula |
Retindex |
|
1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester |
278 |
C16H22O4 |
1908 |
|
1,2 Benzenedicarboxylic acid, dibutyl ester |
278 |
C16H22O4 |
2037 |
|
1,2-Benzenedicarboxylic acid, butyl 2-methylpropyl ester |
278 |
C16H22O4 |
1973 |
|
Phthalic acid, hex-3-yl isobutyl ester |
306 |
C18H26O4 |
2107 |
|
Phthalic acid, 4,4-dimethylpent-2-yl heptyl ester |
362 |
C22H34O4 |
2484 |
|
Phthalic acid, 2,4-dimethylpent-3-yl heptyl ester |
362 |
C22H34O4 |
2441 |
|
Bis(2-ethylhexyl) phthalate |
390 |
C24H38O4 |
2704 |
|
Phthalic acid, di(2-propylpentyl) ester |
390 |
C24H38O4 |
2704 |
|
Phthalic acid, di(6-methylhept-2-yl) ester |
390 |
C24H38O4 |
2575 |
|
Phthalic acid, 2-ethylhexyl tetradecyl ester |
474 |
C30H50O4 |
3364 |
|
Phthalic acid, octyl 2-propylpentyl ester |
390 |
C24H38O4 |
2768 |
Table 3 Chromatogram of Phthalic acid, di(2-propyl pentyl) ester, using gas chromatography and mass spectroscopy gc/ms method of analysis (Mass Peaks: 395,400)C3
Table 4 Shows the Chromatogram E3 of Gas chromatography and mass spectrometry (GCMS) of compound E3. The pure extract contains arrays of compounds, which listed as follows; 2-Naphthalenol, 1,2-dihydro-, acetate(1,2-Dihydro-2-naphthalenyl acetate)(C12H12O2), Azulene (Bicyclo[5.3.0]decapentaene, Cyclopentacycloheptene $$Azunamic, Bicyclo(5.3.0)-1,3,5,7,9-decapentaene(C10H8), Naphthalene (Albocarbon ,Dezodorator, Moth flakes, Naphthalin, Naphthaline Naphthene, Tar camphor, White tar(C10H8), N-Methyl-9-aza-tricyclo [6.2.2.0(2,7)]dodec-2,4,6,11-tetraene-10-one(C12H11NO), 1H-Indene, 1-methylene-(1-Methylene-1H-indene)( C10H8), Dodecane (C12H26), Undecane (C11H24), Octane, 2,3,7-trimethyl-(2,3,7-Trime thyloctane)( C11H24), Dodecane, 2,7,10-trimethyl-, 2,7,10-Trimethyldodecane(C15H32), 1,4-Methanonaphthalene, 1,4-dihydro Benzonorbornadiene $$ 1,4-Dihydro-1,4-methanona phthalene(C11H10), Benzocycloheptatriene (5H-Benzo[a]cycloheptene , 3,4-Benzotropilidene)( C11 H10),1H-Indene,1-ethylidene- (1-Ethylidene-1H-indene, Indene, 1-ethylidene-)(C11H10), Eicosane, 10-methyl- (10-Methylicosane(C21H44), Nonadecane, 2-methyl- 2-Methylnonadecane(C20H42), 9-(2',2'-Dimethylpropanoilhydrazono)-3,6-dichloro-2,7-bis-[2-(diethylamino)-ethoxy] fluorine, N'-(3,6-Dichloro-2,7-bis[2-(diethylamin(C30H42Cl2N4O3), Dibutyl phthalate (1,2-Benz enedicarboxylic acid, dibutyl ester, Phthalic acid, dibutyl ester, n-Butyl phthalate , Butyl phthalate)(C16H22O4), Hexacosa-2,25-dione (2,25-Hexacosanedione)(C26H50O2), Octadecanal, 2-bromo-(2-Bromoo ctadecanal)( C18H35BrO), 1-Pentacontanol(C50H102O), Pentadecane, 2,6,10,14-tetramethyl-(Bute hydrocarbon, Norphytan, Norphytane, Pristan, Pristane,2,6,10,14-Tetram ethyl) (C19H40),1-Butanol, 3-methyl-, benzoate(Isopentyl alcohol, benzoate, Benzoic acid isoamyl ester)(C12H16O2), Benzoic acid, 2-ethylhexyl ester(Ethylhexyl benzoate)( C15H22O2).
|
Name |
Molecular weight |
Structural formula |
Retindex |
|
|
2-Naphthalenol, 1,2-dihydro-, acetate |
188 |
C12H12O2 |
1476 |
|
|
AzuleneBicyclo[5.3.0]decapentaene |
128 |
C10H8 |
1069 |
|
|
Naphthalene |
128 |
C10H8 |
1231 |
|
|
9-(2',2'-Dimethylpropanoilhydrazono)-3,6-dichloro-2,7-bis-[2-(diethylamino)-ethoxy]fluorene |
576 |
C30H42Cl2N4O3 |
4330 |
|
Table 4 Chromatogram C30H42Cl2N4O3 - 9-(2',2'-Dimethylpropanoilhydrazono)-3,6-dichloro-2,7-bis-[2-(diethylamino)-ethoxy ]fluorene) using gas chromatography and mass spectroscopy gc-ms method of analysis (Masspeaks: 395,400)E3
Table 5 shows the Chromatogram F3 of Gas Chromatography and Mass Spectrometry (GCMS) of compound F3. The pure extract contains arrays of compounds, which listed as follows; Dodecane, (C12H26), Bis(2-ethylhexyl) phthalate (Phthalic acid, bis(2-ethylhexyl) ester, Bis(2-ethylhexyl) 1,2-benzenedicarboxylate,Bisoflex 81(C24H38O4), Eicosane, 10-methyl-(C21H44), Nonadecane(C20H42), Phthalic acid, hex-3-yl isobutyl ester(C18H26O4), Heptane, 5-ethyl-2-methyl-(5-Ethyl-2-methylheptane, Heptane, 3-ethyl-6-methy)( C10H22), Dibutyl phthalate (C16H22O4), Hexadecane, 2,6,10,14-tetramethyl- (Phytan, Phytane, 2,6,10,14-Tetramethylhexadecane)(C20H42), 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester(C16H22O4), Pentadecane, 2,6,10,14-tetramethyl- Butehydrocarbon(C19H40), Eicosane(C21H44), Aspidofractinine-3-methanol, (2.alpha.,3.beta.,5.alp ha.)-(C20H26N2O), 1,4-Methanonaphthalene, 1,4-dihydro-(Benzonorborna diene,1,4-Dihydro-1,4-methanonaphthalene (C11H10), Benzocycloheptatriene(C11H10), (Figures 2–6).
|
Name |
Molecular weight |
Structural formula |
Retindex |
|
Dodecane |
170 |
C12H26 |
1214 |
|
Undecane |
156 |
C11H24 |
1115 |
|
Bis(2-ethylhexyl) phthalatePhthalic acid, bis(2-ethylhexyl) ester |
390 |
C24H38O4 |
2704 |
|
Eicosane, 10-methyl- |
296 |
C21H44 |
2045 |
|
Nonadecane, 2-methyl- |
282 |
C20H42 |
1945 |
|
Hexadecane, 2,6,10,14-tetramethyl- 2,6,10,14-Tetramethylhexadecane |
282 |
C20H42 |
1753 |
|
Terephthalic acid, dodecyl 2-ethylhexyl ester |
446 |
C28H46O4 |
3165 |
Table 5 Chromatogram Terephthalic acid, dodecyl 2-ethylhexyl ester, using gas chromatography and mass spectroscopygc/msmethod of analysis (Masspeaks:395,400) F3
The antibacterial effect of the purified compounds on selected clinical Gram negative (E.coli), Gram-positive (Bacillus subtilis) and (Aspergillusflavus) at 10,5,2.5 and 1.25g/mLis presented in Table 6. All the compounds showed remarked inhibitory effect on the selected organisms. Compound A1 has the highest zone of inhibition of 28.0 mm at 10g/mL against E. coli while, Compounds E3 and F3 did not show any inhibitory effect on the bacterium at all the concentrations used. All the compounds showed significant inhibitory effects on Bacillus subtilis and Aspergillus flavus at all the concentrations except F3 at concentration of 1.25 g/mL. Of all the compounds, B3 has the highest zone of growth inhibition of 30.0mm against E. coli. There was significant difference in the diameters of zones of growth inhibition of the compounds on the organisms (p < 0.0001).
|
Compound |
E.coli |
|
|
|
Bacillus |
subtilis |
|
Aspergillus flavus |
|
|
||
|
Concentration (µg/ml) |
10 |
5 |
2.5 |
1.25 |
10 |
5 |
2.5 |
1.25 |
10 |
5 |
2.5 |
1.25 |
|
A3 |
24 |
15 |
9 |
7 |
23 |
10 |
5 |
2 |
24 |
18 |
10 |
6 |
|
B3 |
30 |
23 |
10 |
7 |
17 |
15 |
10 |
8 |
23 |
18 |
10 |
7 |
|
C3 |
22 |
19 |
5 |
3 |
18 |
12 |
5 |
2 |
20 |
17 |
10 |
9 |
|
E3 |
0 |
0 |
0 |
0 |
17 |
17 |
10 |
2 |
8 |
5 |
2 |
0 |
|
F3 |
0 |
0 |
0 |
0 |
22 |
18 |
2 |
0 |
17 |
16 |
0 |
0 |
Table 6 Antimicrobial effect of essential oil purified compounds on selected test bacteria and fungus
Table 7 shows the synergetic antibacterial activities of the purified compounds on the selected organisms at 10, 5, 2.5, 1.25g/ml. There was marked inhibitory effect observed in all the combined compounds with the highest zone of growth inhibition (36.0mm) recorded against E. coli with combination of Compounds A1 and A3. Combined compounds A1+ A3, B3+C3and E3 +F3 showed diameters of inhibition of 32.0mm, 30.0mm and 20.0 mm against Aspergillus flavus, respectively. There was significant difference in the diameters of zones of growth inhibition of the compounds on the organisms (p < 0.0001).
|
Compound |
E.coli |
|
|
|
Bacillus subtilis |
|
Aspergillus flavus |
|
||||
|
Concentration µg/ml |
10 |
5 |
2.5 |
1.25 |
10 |
5 |
2.5 |
1.25 |
10 |
5 |
2.5 |
1.25 |
|
C3+ A3 |
36 |
31 |
25 |
16 |
30 |
28 |
19 |
14 |
32 |
29 |
20 |
15 |
|
B3 + C3 |
32 |
29 |
27 |
18 |
20 |
17 |
12 |
10 |
30 |
20 |
16 |
8 |
|
E3 + F3 |
30 |
26 |
15 |
10 |
25 |
17 |
14 |
12 |
20 |
15 |
10 |
5 |
|
A3 + B3 |
28 |
26 |
20 |
15 |
27 |
20 |
18 |
15 |
18 |
15 |
10 |
6 |
Table 7 Synergetic antimicrobial effect of purified compounds on selected test bacteria and fungus
Key: C3+ A3, Phthalic acid, di(2-propyl pentyl) ester+Aspido fractinine-3-methanol,(2.alpha.,3.beta.,5.alpha);
B3 + C3, Phthalic acid, 2-ethylhexyl tetradecylester +Phthalic acid, di(2-propyl pentyl) ester; E3 + F3, 9-(2',2'-Dimethyl propanoilhydrazono)-3,6-dichlo ro-2,7-bis-[2 (diethylamino)-ethoxy ]fluorene + Terephthalic acid, dodecyl 2-ethylhexyl ester; A3 + B3, Aspido fractinine-3-methanol,(2.alpha.,3.beta.,5.alpha)+Phthalic acid, 2-ethylhexyl tetradecyl ester.

Figure 2 Structural profiling compound with the highest peak (A3):C20H26N2OAspido fractinine-3-methanol, (2.alpha.,3.beta.,5.alpha).

Figure 3 Structural profiling compound with the highest peak; (B3) C30H50O4 Phthalic acid, 2-ethylhexyl tetradecyl ester.

Figure 4 Structural profiling compound with the highest peak (C3) C24H38O4Phthalic acid, di (2-propylpentyl) ester.
The purpose of this research work is to isolate, purify, chemical identification, antimicrobial and synergistic efficacy of extracted essential oils fromethyl acetate extract of Spondias mombin. Plants produce a variety of compounds with antimicrobial activity. Some are always present while others are produced in response to microbial invasion.20–27 Identifying the most active antimicrobial compounds of essential oils is cumbersome because essential oils are complex mixtures of up to 30 different constituents, 23–25 and the composition of a particular essential oil may vary depending on the season of harvest, and the methods used to extract the oil.26 The antimicrobial activity of Essential oil (EOs) of Spondias mombin, similar to all natural extracts is despondent on their chemical compositions and the amount of the single component. Many of the plant antimicrobial compounds are constitutively expressed by the plant and others can be synthesized as mechanism of self-defense in response to pathogens. Vegetable, spices and fruits like Spondias mombin, fruits with high level of EOs are excellent sources of natural compounds with activity against microorganism.15
In this study, ethylacetate was used to extract the Stem bark extract of S. mombin because most plant constituents are stored in vacuoles in the plant and are commonly extracted into solvents since it retains sufficient hydrophilicity to extract intra cellular (water-soluble) and extracellular compounds. 16,17 The collected plant materials were air dried under shade before extraction. Higher temperature and sun drying were avoided to prevent degradation and decomposition of constituents that may be sensitive to extreme heat and light. After extraction, the filtrates were concentrated in vacuo. The crude extracts were stored in air tight containers to prevent microbial growth. The antimicrobial mode of action of essential oil observed in this study constituents have been performed on bacteria and fungi while little is known about their action on fungal (yeast and molds). Gram-negative bacteria are generally less susceptible than Gram-positive bacteria. 28 The outer membrane of Gram-negative bacteria contain hydrophilic lipopolysaccharides (LPS), which create a mechanical barrier toward macromolecules and hydrophobic compounds, providing Gram-negative bacteria with higher tolerance toward hydrophobic antimicrobial compounds like those found in essential oils under the scope of this research work. 29 Antimicrobial compounds that act on the membrane can cause depolarization or increased permeability through various mechanisms. For example, the extracted essential oil from S. mombin, some antimicrobial peptides form pores 30,31 while other compounds, such as certain essential oil constituents, have a fluidifying effect on the membrane.
From the results of the assessment of antimicrobial activities of the pure isolated compounds on selected test organisms (Table 6). It was revealed that all the pure extracts possessed marked antimicrobial activities against the test organisms. Similarly, the synergistic activities of these compounds (Table 7) results in significant antimicrobial activities recorded against the selected test organisms. This may be due to the synergistic effects of the combined compounds.
A method for preliminary fractionation of complex plants is by fractionation using vacuum liquid chromatography with solvents of various degrees of polarity. Each of the solvent which separates the components of extracts based on approximate polarity would result in distribution of the active constituents throughout several fractions. This principle was applied to the crude extract of the plant and was successively extracted with solvents and mixture of solvents with varying polarity. Each fraction was concentrated to dryness in vacuo. Thin-layer chromatography (TLC) analysis showed that the profiles of the fractions were distinctly different. Thus vacuum liquid chromatography was a good method for the preliminary fractionation of the crude extracts of the plant. It also served as a general purpose clean up method such that the compounds were obtained with high degree of purity. The compounds were characterized using Gas Chromatography-Mass Spectroscopy.
Most of the compounds identified by GCMS are essential oils (Tables 3–5). This may contribute to the observed antimicrobial activity S. mombin recorded against the susceptible test bacteria and fungi in this study. Aspidofractinine-3-methanol, Phthalic acid, 2-ethylhexyl tetradecyl ester, Phthalic acid, di(2-propylpentyl)ester),9-(2',2'-Dimethylpropanoilhydrazono)-3,6-dichloro-2,7-bis-[2 iethylamino)ethoxy]fluorine); Terephthalic acid, dodecyl 2-ethylhexyl ester are basically the compound isolated from the Spondias mombin. Aspidofractinine-3-methanol are Alcohol with Antimicrobial, antifungal activity,22 Phthalic acid, 2-ethylhexyl tetradecyl ester), Ethyl, tetra, hexyl, decyl ester-Benzene-dicarboxylic acid diethyl ester has antimicrobial, antioxidant and hypocholesterolemic properties,30 Phthalic acid, di(2-propylpentyl) ester are bicyclic aromatic hydrocarbon ester with anti-inflammatory, antimicrobial properties (31), 9-(2',2'-Dimethylpropanoilhydrazono)-3,6-dichloro-2,7-bis-[2-(3,6-dichloro-2,7-bis-[2-(diethylamino)-ethoxy]fluorine) are basically Fatty acid with antimicrobial, antioxidant, cytotoxic, antifungal and antitumor properties,29 Terephthalic acid, dodecyl 2-ethylhexyl ester), Benzene-dicarboxylic acid diethyl ester has antioxidant, hypocholestero-lemicactivity.18,32
Spondias mombin essential oil can affect both the external envelope of the cell and the cytoplasm, It should be noted that the compounds identified in this study like Benzoic acid and 2-pipondinone, phthalate, 7, 8, 9, octadecanal, Diisotyl phthalate have been reported to have permeability barrier provided by the cells membrane which is indispensable to many cellular functions including maintaining the energy status of the cell, membrane coupled energy transducing process, solute transport and metabolic regulation.19,20 Spondias mombin essential oil has been reported to inhibit the growth of bacterial cellsand also inhibit the production of toxic bacterial metabolites, and appears to be more effective against Gram positive bacteria than Gram negative species; the effect which may likely due to differences in the cell membrane composition.21 From the results of the assessment of antimicrobial activities of the pure isolated compounds on selected test organisms (Table 6). It was revealed that all the pure extracts possessed marked antimicrobial activities against the test organisms.
Osuntokun reported the role of essential oils in the discovery of new drugs cannot be overemphasized, especially in an era of antimicrobial resistance. With essential oils, one may suggest that certain abundant and effective constituents such as thymol, β-carophyllenes and eugenol as major highlights so far for development of potent antimicrobials. This is due to the fact that they possess novel chemistry which could offer a remarkable opportunity for discovery of new drugs,many antimicrobial resistant organisms including methicillin resistant staphylococcus aureus (MRSA) can be controlled using essential oils. Researchers are therefore encouraged to tailor drug design towards the use of essential oils (especially eugenols and thymols) to curb antimicrobial resistance. Other pharmaceutical potentials in the treatment of infection.
The synergistic activities of these compounds (Table 7) results in significant antimicrobial activities recorded against the selected test organisms. This may be due to the synergistic effects of the combined compounds, for example, Phthalic acid, di (2-propyl pentyl) esterand Aspido fractinine-3-methanol,(2.alpha.,3.beta.,5.alpha),Phthalic acid, 2-ethylhexyl tetradecyl ester and Phthalic acid, di (2-propyl pentyl) ester, 9-(2', 2'- Dimethyl propanoilhydrazono)-3,6-dichlo ro-2,7-bis-[2(diethylamino)-ethoxy ]fluorineand Terephthalic acid, dodecyl 2-ethylhexyl esterAspido fractinine-3-methanol, (2.alpha.,3.beta.,5.alpha) and Phthalic acid, 2-ethylhexyl tetradecyl ester. All isolated ester exhibits different degree of antimicrobial syngistic activity from the ethyl acetate stem bark extract of Spondiasmombin due to combination of different array of essential oil.30
In conclusion, Spondias mombin is a unique medicinal plant with various medicinal purposes, and the essential oil extracted from the ethyl acetate stem bark has proven to be a potent therapeutic and antimicrobial agents in the annals of nature’s chemistry in drug discovery and treatment of various infectious diseases due to the fact that Spondias mombin possess different novel compound and itsynergized with different novel compound which has become an indomitable force of nature in the treatment of infectious diseases.
None.
The author declares there is no conflicts of interest.
None.

©2019 Osuntokun, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.