International Journal of
eISSN: 2381-1803


Research Article Volume 11 Issue 1
1Institute of Tissue Banking and Biomaterial Research, Atomic Energy Research Establishment, Savar, Bangladesh
2Department of Biochemistry & Molecular Biology, Jahangirnagar University, Bangladesh
Correspondence: SM Asaduzzaman, Institute of Tissue Banking and Biomaterial Research, Atomic Energy Research Establishment, Savar, Dhaka-1349, Bangladesh, Tel +8801726894690
Received: November 01, 2017 | Published: January 23, 2018
Citation: Hossain ML, Islam MM, Diba F, Hasan MZ, Asaduzzaman SM (2018) The Synergistic Effect of AM and MO Derived Gel in Burn and Wound Healing. Int J Complement Alt Med 11(1): 00341. DOI: 10.15406/ijcam.2018.11.00341
Burn is a major clinical concern in the developing and poor countries. Now-a-days the treatment of burn in an effective way is a great challenge. It has already been established that amniotic membrane and Moringa oleifera, a medicinal plant, are a great focus of interest for burn wound healing. Amniotic membrane is a natural gift which promotes faster wound healing in partial and superficial burn injury since 1910. It has prominent characteristics in the management of burn injury such as availability, economical concern, faster wound healing, pain reduction and provide protection from the infection. Furthermore,Moringa oleifera also promotes wound healing process, wound contraction and increases the rate of epithelialization due to their astringent and antimicrobial properties and the bioactive compound vicenin-2. As the burn healing process is time consuming, recently patients are suffering from the various difficulties such as slow epithelialization rate, poor dressing, staying in the hospital for long time. Therefore, the focus of this review is to explore the combined effects of amniotic membrane and Moringa oleifera for faster wound healing in the management of burn patient.
Keywords: Wound and burn injury; Amniotic membrane; Moringa oleifera; Combined effect; Faster healing
AM, amniotic membrane; MO, Moringa oleifera; ITBBR, institute of tissue banking and biomaterial research; VEGF, vascular endothelial growth factor; HGF, hepatocyte growth factor; TGF, transforming growth factor; HLA, human leukocyte antigen; DR, differential regulation; MSC, mesenchymal stem cells; SLPI, secretary leukocyte proteinase inhibitor; AECS, amniotic epithelial cells; AMCS, amniotic mesenchymal cells
Skin is the largest organ in body which comprises 15% of the body weight. It serves as a wall-like barrier that protects our body from the environmental microorganisms.1,2 Epidermal glands in the skin secrete substances which contribute to the preservation of water-electrolyte balance of the body and permit water vapor. It also regulates body temperature through neural feedback mechanism by operating the hypothalamus and is formed by two main layers, including epidermis covering the body surface and the dermis involving the connective tissue.3 Skin can be injured basically by two types of wounds: (i) open wounds where skin is torn, cut or punctured and (ii) closed wounds where blunt force trauma causes a contusion. Among various kinds of wounds, burn is considered one of the most severe wounds as they are susceptible to infections due to vascular necrotic tissue. Epidermal integrity loss in the skin structure is the foremost consequence of burn wound. Thermal, electrical and chemical injuries are the most common reasons of burn wounds. The severity of burn injuries is set according to the depth of skin involvement and the percentage of total body surface area.4 Skin burn wound is a fourth graded injury among all injuries. In spite of recent advances in burn wound treatment, scarring rate remains high and the deaths are over 180,000 each year throughout the world (WHO, 2017).5 Currently, burn death rates are decreasing in many high-income countries but the rate of child deaths from burns is over 7times higher in low- and middle-income countries. Since burns have become a global public health problem, to treat burn patients several anti-burn drugs are used. However, these drugs have many side effects and pose an economic burden to the patients. Therefore, scientists have turned to natural remedies, including human amniotic membrane (AM) and other wound healing plants. Among these plants Moringa oleifera (MO) is one of them which have many evidences on the wound healing activities. Although there have been many research activities on both AM and MO in burn wound healing, their combined effect has not been yet observed. If a gel combined with AM and MO leaves is prepared, the combined effect may be very beneficial for burn wound healing.
The inner most lining of the human placenta that is normally discarded after parturition is amniotic membrane. It contains many growth factors, proteins and stem cell reserves that accelerate wound healing. The amnion is expanded and become the amniotic sac due to the amniotic fluid inside it which serves a protective environment for embryo development. It is the nearest thing to the epidermis and it is like an extension of the baby's skin that is formed by the ectoderm of the fetus.6
Amniotic membranes develop from extra-embryonic tissue and consist of a foetal component (chorion and amnion) and a maternal component (deciduas) (Figure 1). These two parts are held together by the chorionic villi and connect the cytotrophoblastic shell of the chorionic sac to the decidua basalis. The foetal component separates the foetus from the endometrium. The amniochorionic membrane forms the outer limits of the sac that encloses the foetus, while the innermost layer of the sac is the AM. Thickness of normal AM is in between 0.02-0.05mm.7 From histological perspective, AM is an avascular, thin, tough transparent membrane.8 It is composed of three major layers: (i) epithelium, a single layer of metabolically active cuboidal and columnar cells which are in direct contact with amniotic fluid; (ii) basement membrane; (iii) and a mesenchymal layer.9,10 The mesenchymal layer is further divided into compact, fibroblast and spongy layer. AM contains two distinct types of cells with different embryonic origin: embryonic ectoderm derived amniotic epithelial cells (AECs) and mesoderm derived amniotic mesenchymal cells (AMCs).11,12
The major components that contribute to the integrity of AM and its biochemical properties are:
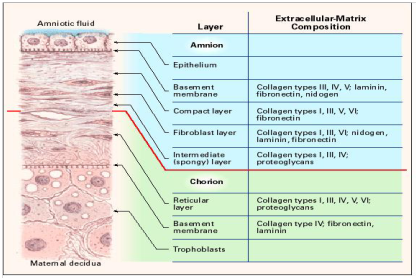
Figure 2 Cross section of the human amniotic membrane indicating the biochemical characteristics of the section. Adapted from Niknejad14 with permission.
Human amnion is used for centuries as a biological burn dressing material to treat superficial- and partial- thickness burns worldwide.15 An ideal wound dressing should be readily available, economical, easy to apply, able to provide good pain relief, able to protect the wound from infection, promote healing, prevent heat and fluid loss, elastic, adhere well to the wound, robust, able to withstand shear force, durable and non-antigenic, but multitudes of burn wound dressings available on the market are unable to meets all these demands.16 AM is almost always discarded but satisfy most of the criteria for an ideal biological dressing. Its efficacy has been proved as a true biological burn dressing material and is readily available worldwide.17,18 It is thin, elastic, adheres to the wound surface well, has effective vapor barrier, a durable cover for the raw surface, and a substantial pain reliever.19‒21
Strong wound adherence property
Amnion very firmly adheres to wound beds. Due to the fibrin-elastin formation at the wound-dressing interface, the bonding between amnion and wound surface is biological, not mechanical.22
No rejection phenomenon
When the amnion is directly placed over the wound surface, it can fulfill some of the functions of the skin that are destroyed in burn injuries. As AM derived from the fetal ectoderm, it has the advantages of fetal skin allograft; furthermore, it is different from skin allograft and does not occur vascularization and rejection of the membrane.20,23 Human amniotic epithelial cells have lower inflammatory response and lack of rejection during allograft because they are unable to present human leukocyte antigen (HLA) A, B, C and DR surface antigens and beta-2-microglobulin.24 Specially the sterilized, freeze-dried AM is biocompatible and retains most of the physical, biological, and morphologic characteristics, so it is a useful biomaterial (Figure 3).
Antimicrobial and antiviral properties
AM seals the wound and prevents contamination via fibrin and elastin linkages.25 This tight bonding protects circulating phagocytes and allows faster removal of surface debris and bacteria from the wound.19 The mesenchymal stem cells (MSC) in AM show its antimicrobial activity in two ways: directly via the secretion of antimicrobial factors such as LL-37 and indirectly via the secretion of immunomodulative factors which upregulates bacterial killing and phagocytosis by immune cells.26,27 It also carries many bactericidal products of purine metabolism and lysozyme including defensins, elafin and secretary leukocyte proteinase inhibitor (SLPI) which act as a component of the innate immune system to provide protection from infection. Fetal skin and AM abundantly contain Cystatin E, which acts as a powerful antiviral agent.28 The mechanism of accelerated wound healing by amnion membrane is shown below (Figure 4).29
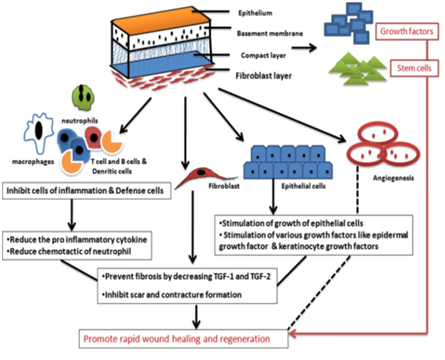
Figure 4 The wound healing mechanism by amniotic membrane. Adapted from Chopra.29
Anti-scarring
AM prevents scarring by secreting endothelial growth factor (VEGF), hepatocyte growth factor (HGF) which help to keep balance between TGF-1 and TGF-3 and down-regulate TGF-beta. It also modulates wound healing by tissue reconstruction29,30 and prevents pathological remodeling and excessive fibrosis by suppressing various immune cells such as T cell, dendritic cell and B cell.30
Pain reduction
AM alleviates pain by diminishing inflammation as well as providing better state of hydration that appease the wound bed to promote faster wound. Application of amnion to burn surfaces can protect the exposed nerve endings from external irritants that helps to decrease pain sensation by preventing nerve stimuli and thus AM reduces the pain score in adults and quieted a distressed child.29,30
Minimizing fluid/water loss
The amnion is composed of five distinctive layers namely epithelial, basement membrane, connective tissue, fibroblasts and a spongy layer. This structure resembles the stratum corneum which is a natural vapor barrier.14 The vapor barrier of AM reduces the amount of fluid loss by preventing excessive evaporation from the wound surface directly.31 However, it is noted that the membrane does not influence the fluid shift from the plasma into the interstitial compartment in the extracellular space. A study conducted by pigeon reported the beneficial role in the early treatment of burns to prevent fluid or plasma leakage.6
Less frequent dressing and faster wound healing
In a comparative study between amniotic membrane and silver sulfadiazine in second degree burn treatment, Mostaque and Rahman showed that the amniotic membrane is a better treatment option because its use reduces the length of hospital stay and the number of required dressing changes. Because of faster epithelialization it is easy and comfortable both for the doctor and patients.32,33 Amnion speeds up the epithelialization process, which results in faster wound healing in superficial and partial-thickness burns (with minimal oozing, and healing occurs without the need for auto-grafting) and superior to both allograft and xenograft materials.20,34 By accelerating the migration of fibroblasts and the development of collagen, amniotic membrane promotes healing during the first 6–8days of repair.34‒3
Neovascularization
The most remarkable effect of amnion is the development of new vessels and it was observed grossly, histopathologically and immunohistologically.37 Increased vascularity in the AM-treated burn wound was noted microscopically.38 The proteins produced by amniotic epithelial cells have angiogenic effects.39
MO is commonly known as drumstick tree which belongs to the family of Moringaceae (Figure 5). It is native in India, Pakistan, Afghanistan, Bangladesh as well and now-a-days, it has been distributed in many countries of tropics and subtropics. It is a miracle tree because it has outstanding medicinal properties including wound healing, anti-tumor, anti-hepatotoxic, anti-fertility, hypotensive, diuretic, anti-ulcer, cardiovascular anti-cancer properties.40‒42
Medicinal properties
Almost every parts of this plant used for various indigenous medicine elements. Because of its chemical constituents and amazing clinical uses (Table 1),40 it has recently been accepted as a demanding medicine.43
Plant part |
Chemical constituents |
Use |
Leaves |
Benzyl iso thiocynate, nlazimicin, benzyl glycolsinolate Anti-oxidant rhamnosloxy) benzyl] isothiocyanate,4-[(3’-O-acetyl-alphai-rhamnosyloxy)benzyl]isothiocyanate and S-methyl-N{4-[(alpha-I-rhamnosyloxy) benzyl]} thiocarbamate |
Anti-microbial anti-bactirial
Anti-ulcer
Anti-inflammatory Anti-diabetic |
Leaves, Seeds, Roots |
Quercetine, Kaempferol |
Anti-oxidant |
Table 1 Chemical constituents and clinical uses of Moringa oleifera
Wound healing mechanism of Moringa oleifera leaves extract
Different complex processes such as inflammation, epithelialization, angiogenesis, granulation, tissue formation and the deposition of interstitial matrix are involved for tissue repair and wound healing.44 Microorganisms, especially bacteria (S. aureus and P. aeruginosa) are responsible for inflammatory response in both acute and chronic wounds and delay the wound healing process. The aqueous fraction of MO leaves contains some phytochemical compounds such as alkaloids, triterpenoids, tannins, and flavonoids. These metabolites possess compounds that play active role in wound healing and inhibits microbial growth by binding with DNA of the microorganisms. Flavonoids and tannins improve wound healing through its antioxidant activity and protect tissues against oxidative damage.45‒47 The astringent and antimicrobial properties of MO leaves promote the wound healing process, wound contraction and increased rate of epithelialization.48 Furthermore, the methanolic crude extract and aqueous fraction of MO contain some bioactive compounds such as; kaempferol, quercetin and vicenin-2. These bioactive compounds are used in the drug discovery and development of wound healing agent that may enhance wound healing.49 The wound healing mechanism of aqueous fraction of Moringa oleifera is shown below (Figure 6).50
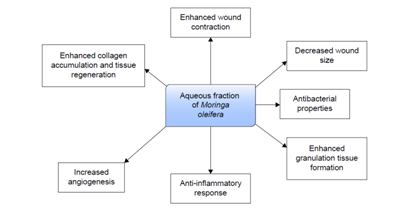
Figure 6 Possible mechanism of aqueous fraction of Moringa oleifera in wound healing. Adapted from Muhammad50 with permission.
Previous reports on the effect of amniotic membrane used with other substances
Several epidemiological studies using animal models have shown that the combined effect of AM is more effective in burn and wound healing. Yifeng K et al.51 conducted a study with 200 rats and generated corneal alkali burn in the right eye of each rat and reported that (elaboration of MSC) MSCs and polysaccharide treatments significantly enhanced the recovery of corneal epithelium; the combination of polysaccharide-MSCs (PM) groups showed additive effects compared with single treatment groups.51
In another study, Patil SD and Rasika J reported the antimicrobial activity of MO with Cleome viscosa. This study was carried out against five pathogenic bacteria where the combination of MO and Cleome viscosa exerted strong synergistic effect towards K. pneumonia, E. coli, S. pneumoniae and S. aureus compared to the single treatment.52 Therefore, they speculated that the combined chemicals may give strong synergistic effect compare to the effect of the single one.
Burn injury is one of the most devastating phenomena in the world. Humans have known the tragedy of severe burn injuries since the ancient time. It is the most common traumas in the developing countries and most burn injuries consume large amount of medical resources. But it is hardly possible to bear the enormous cost for the people with lower socio-economic classes. So it is important to find an appropriate material for dressing of burn wounds that improves healing and is readily available, easily applicable, economical, protective from infection and desiccation, and facilitates healing.53 Both AM and MO fulfil the above criteria. AM is being widely used as burn wound healing material worldwide and known as an ideal biological burn wound dressing material.17,18,23 Besides, MO is such a plant that all the parts of this plant e.g. leaf, blooms, root, bark, gum, seed and seed oil have been used for different ailments as a part of the indigenous medication of South Asia, including the treatment of inflammation and infectious diseases.54‒56 Its leaves contain different type of compounds, for example, flavonoids, ascorbic acid, phenolics and carotenoids which are good source of natural antioxidant.57 These phenols have multiple beneficial biological effects including antioxidant activity, anti-inflammatory action, inhibition of platelet aggregation, antimicrobial activities and anti-tumor activities.58 It has huge quantities of proteins (20-29%), vitamins and minerals which play vital role in the defense mechanisms and ultimate wound healing.59‒61 The aqueous extract of MO has wound repairing property in male Swiss albino mice. Rathi BS et al.62 conducted a study with albino rats and observed a significant increase in the reduction of scar and percent wound contraction was reduced that indicated to the healing properties of the aqueous extract of Moringa oleifera leaves.62 A study conducted by Hukkeri VI and his group described antipyretic and wound healing properties of the ethyl acetate and ethanolic extracts of MO leaves.63 Significant antipyretic activity is observed in rats due to the ethanolic and ethyl acetate extracts of seeds where ethyl acetate extract of dried leaves shows wound healing activity (10% extracts in the form of ointment) on excision, incision and dead space (granuloma). After all, bioactive fraction of MO shows antimicrobial, anti-fungal activities and vicenin-2 compound enhances faster wound healing.49
In our study we found that both AM and MO are crucial agents for faster wound healing, better epithelialization, no rejection phenomena, less patients suffering and more importantly economical for poor patients. Since, freeze dried irradiated amniotic membrane and irradiated powdered Moringa oleifera leaves have no antigenicity, a gel combined with them could have used to speed up burn wound healing with cheaper rate. If it is possible, it might be a new frontier for burn patients specially those are very poor. In our following study the extensive research works will be performed to evaluate the combined effects of AM and MO derived gel, expecting more effectivity and beneficial role in the wound and burn healing.
Md. Liakat Hossain carried out study design, literature citing, table and figure collection, editing and manuscript writing. Md Majharul Islam helped to conduct literature citing, table and figure collection and manuscript writing. Farzana Diba contributed to literature citing and manuscript reading. Md. Zahid Hasan designed study and participated in helping to edit, revise and approve the final manuscript. S. M. Asaduzzaman supervised the writing of the manuscript and revised it critically for important intellectual content. All authors read and approved the final manuscript.
I would like to extend my sincere thanks to all authors who collaborate this work. The current study was derived from an MSC thesis conducted by me.
The authors declare that there is no conflict of interest.

©2018 Hossain, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.