International Journal of
eISSN: 2381-1803


Short Communication Volume 14 Issue 6
1 Department of Drugs and Medicine, SUniversity of São Paulo State” (UNESP), Brazil
2 Department of Physiology and Pathology, University of São Paulo State” (UNESP), Brazil
3 Advanced Research Center in Medicine, School of Medicine, Union of the Colleges of the Great Lakes (UNILAGO), Brazil
Correspondence:
Received: September 02, 2021 | Published: November 10, 2021
Citation: Jornada DH, Chiba DE, Scarim CB, et al. The preventive effect of Taurine in early stage of colon chemical carcinogenesis. Int J Complement Alt Med. 2021;14(6):242-244. DOI: 10.15406/ijcam.2021.14.00568
Colorectal cancer is one of the most common types of cancer in the world. The aberrant crypts foci are the first dysplastic alterations observed in colons in animal models and in human beings. Taurine is the most abundant semi-essential β-amino acid in mammals and is reported to have a broad anti-inflammatory and antioxidant activity. The purpose of this work was to demonstrate the protective effect of taurine in colon cancer model using the 1,2-dimethylhydrazine (DMH) as a pro-carcinogen agent. For this, the induction of carcinogenesis processes in the Wistar male rats were obtained by the administration of DMH (40mg/kg/day, subcutaneous, twice a week) for two weeks. On the day following the induction, the animals started receiving the taurine (gavage, 100mg/kg/day) for 15 days. Then, the colon was removed, opened longitudinally and ACF (aberrant crypt foci), AC (aberrant crypts) and multiplicity (ACF/AC) were evaluated. The results showed that taurine promoted the decrease of multiplicity of the crypts in the proximal colon (59%) when compared to the non-treated group. The data point to a chemopreventive action of taurine in the promotion of colon cancer in early stage, mainly in the proximal portion which justify further studies to clarify the mechanisms and therapeutic uses.
Keywords: taurine, chemoprevention, early colon carcinogenesis, aberrant crypt
Colon and rectum cancer is the second most frequent reached 1,93million cases and cause 935.000 death worldwide in 2020.1 The early stage of colon cancer chemotherapy remains a challenge that consists in a surgery followed by adjuvant chemotherapy to target the eradication and cure.2 Initiation stage is characterized by exposure to the carcinogenic agent (initiator) and DNA alteration, leading to the production of initiated cells. In the promotion stage, the selective clonal expansion of the genetically altered cells occurs, promoting morphological modifications, as aberrant crypt foci,3,4 considered biomarkers of the tumor initiation process.8 In reality, the crypts are part of the colon, and are formed by cells that produce mucins, but the carcinogenesis process promotes crypt enlargement, wall thickening, cell proliferation, increased crypt formation within the same focus reducing the production of mucins, called as “aberrant” and are viewable under microscopy.5,6 With the propagation of mutation cells, the progression phase occurs, when self-sufficiency and disordered cellular proliferation are observed. This phase is irreversible and leads to the clinical manifestations of the disease.3,6
Colon cancer induction by chemical agents acts by increasing oxygen reactive species and inducing the inflammatory process, causing tissue damages and modification in macromolecules. The 1,2-dimethylhydrazine (DMH) acts as a colon specific pro-carcinogen, since it requires bioactivation to exert its carcinogenic action. Its metabolism occurs in the liver and the subproducts azoxymethane (AOM), methylazoxymethanol (MAM) and methionine ion are transported to the colon along the bile salts, where they damage the colonic mucosa through the formation of adducts with the DNA, promoting cell proliferation, inhibition of apoptosis and induction of the aberrant crypts.7
Taurine (2-aminoethanesulfonic acid) is the most abundant semi-essential β-amino acid in mammals, and has broad anti-inflammatory and antioxidant activity.8-11 Some studies have described the taurine action in tumoral cells. One of them has observed that concentrations of 80 μM can promote apoptosis in cultures of colon tumor cells, proportional to the increase in the concentration of taurine.9 In breast cancer cells, taurine has presented a protective effect, including in cell line modified in p53. In vivo, inhibition of tumor growth and increase of the transcription factor PUMA (p53 upregulated modulator of apoptosis) was observed, promoting the activation of caspase-3 and triggering apoptosis of tumor cells10. Another study has described similar effects in mammary carcinogenesis, where taurine inhibited carcinogenesis through regulation of mitochondrial function, stimulating the production of antioxidant enzymes. Moreover, the amino acid has the ability to inhibit the development of breast tumors through the induction of tumor cell apoptosis mediated by the increase of p53.11 Thus, the purpose of this work study the effect of taurine in the induction and promotion of colon cancer through the carcinogenesis induction by DMH.
Animals and experimental design: The study was approved by Brazilian Committee for the Experimental Use of Animals and the procedures were carried out according to a Technical Regulation of the National Council of Animal Experimentation and the simple-blind assay was constructed based on Silva Almeida et al.12 For this, 23 Wistar rats (104weeks, male), from UNESP, Botucatu, SP, Brazil were maintained under controlled temperature (22°C±2°C), air and light dark cycle 12/12hs, receiving autoclaved rat chow, water, ad-libitum.
The animals were randomly divided by: (POS) positive control (n=7), (TAU) taurine (n=8). POS and TAU have received DMH (40mg/kg/day) (Sigma-Aldrich, USA, pH 6.5 in NaCl solution), four subcutaneous injection/twice a week, for two weeks. At the day after the last administration of DMH the (TAU) group has received taurine (gavage, 100mg/kg/day) (99%, Chem Impex, water) for fifteen days. (NEG) received water. In the sixteenth day all animals were sacrificed (CO2) and the colon was removed for analysis.
Colon processing and ACF (Aberrant Crypt Foci) analyses: The removed colon was opened longitudinally, washed with a saline 0.9% solution and fixed with formalin solution buffered (10%, pH 6.9-7.1) for 48hours and maintained in an ethanol (70%). The colon was measured and divided in three portions, proximal, medial and distal, according to proximity to the small intestine. ACF analyses were performed with a methylene blue 0.1% in light microscope, objective 10X magnification and analyzed according to Bird criteria (2000)6 and the incidence, score of ACF, AC, and multiplicity (AC/ACF) were analyzed in twenty-five consecutive fields.
Statistical analysis: software Graph Pad Prism 6.01 was used. When the data met the normality assumption (Shapiro-Wilk), the comparison between the groups was analyzed by T-test for non-paired measures. Data that did not meet the normality assumption were analyzed using the Mann Whitney test. The data that presented heteroskedastic variance were analyzed through the unpaired T test with Welch correction. The level of significance was 5%.
Animals' deaths and a significate weight variation were not observed after four measured weights during the experiment (data not shown). The incidence of dysplastic crypts was the same in (POS) and (TAU) group. The same has been observed for ACF and AC score in all portions of the colon. However, the relation between AC and ACF (multiplicity) has showed a significative difference (p<0.05) in the proximal colon of (POS) and (TAU), decreasing multiplicity by approximately 59% (Figure 1 and Table 1).
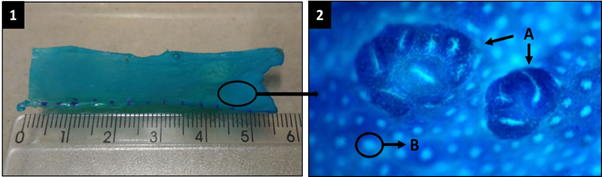
Figure 1 Colon. (a) Macroscopic image; (b) Colon crypt, 10 X (b.A) aberrant crypt foci; (b.B) normal colon crypt.
|
Coloan portion |
Group/treatment |
||
(NEG) |
(POS) |
(TAU) |
||
Dysplastic ACF N (%) |
Total colon |
0 (0) |
7 (100) |
8 (100) |
AC |
proximal colon |
0 |
13.71±3.58 |
5.00±1.85 |
medial colon |
0 |
38.86±5.93 |
24.86±4.80 |
|
distal colon |
0 |
25.57±6.16 |
21.00±5.15 |
|
Total colon |
0 |
78.14±12.02 |
50.86±9.18 |
|
ACF |
proximal colon |
0 |
6.86±1.93 |
3.14±0.88 |
medial colon |
0 |
20.43±3.03 |
14.29±2.80 |
|
distal colon |
0 |
14.86±3.58 |
12.57±2.14 |
|
Total colon |
0 |
42.14±6.84 |
30.00±4.07 |
|
AC/ACF (multiplicity) |
proximal colon |
0 |
2.12±0.25 * |
1.24±0.25 * |
medial colon |
0 |
1.92±0.17 |
1.81±0.23 |
|
distal colon |
0 |
1.68±0.10 |
1.58±0.15 |
|
Total colon |
0 |
5.72±0.30 |
4.63±0.47 |
|
Table 1 Percentage of aberrant crypt, aberrant crypt foci and the multiplicity after taurine (100 mg/kg) treatment in chemical colon cancer
(NEG) no cancer induction/treatment; (POS) cancer induction by DMH (40mg/kg/day), (TAU): cancer induced by DMH and after 15days of treatment with taurine (100mg/kg)
N= total animals; Values are mean±SE
*Statistical difference between lines (p<0.05).
Studies with taurine and cancer have been increasing in the last years. The taurine biological activity against several types of tumors, such as breast, liver, Ehrlich's ascites tumor, and colon has been described, both as chemopreventive effect of carcinogens and as an inhibitor of tumor cell proliferation, used alone or in association8-11,13 In contrast, it was described as a mechanism of promotion of colon cancer, based on the type of human diet and hypothesize that an animal protein diet is rich in taurine that is conjugated in the bile and its metabolism by gut anaerobic microbes produces H2S, a highly genotoxic metabolite and as a consequence, leading to the promotion of colon cancer, concluding that taurine disposition via meat intake is responsible for colon cancer in individuals who consume meat, unlike vegetarians.14 However, this hypothesis did not consider others compounds such as amine biogenic metabolites that can form carcinogenic nitrosamines with nitrite in meat and also fermented foods.15,16Besides that, H2S after taurine supplementation were associated to an antihypertensive effect in prehypertensive patients17 and a recent publication of Zaorska et al.18 reviews the therapeutic effect of H2S and H2S donor compounds as potential gastrointestinal anti-inflammatory drugs useful in colitis.
Derivative of taurine conjugate forms, such as tauroursodeoxycholic acid is able to attenuate colon cancer by inhibition of nuclear factor kappa B (NFk-B) pathway.19 Also, in our previous study, the in vivo antimutagenic effect of taurine promoted by mutagenic drugs (such nitro compounds) was shown between 45-79 %, depending on the drug, by taurine blockade of chromosome, interfering in the cell division or by its antioxidant effect against the nitro bioreduction by-products.20 Our findings corroborate with the results of Wang et al.21 and suggest that taurine can act in the chemoprotection of colon early carcinogenesis, through the reduction of multiplicity of aberrant crypt foci, being considered a biomarker in colorectal cancer progression.22
Interestingly, our results suggest a specific activity in proximal colon, which can be related with taurine solubility and permeation into the colon. Highly soluble in water (weakly liposoluble) at physiological pH, taurine is in its ionized form, requiring transmembrane proteins to be absorbed into the cell (Na+ and Cl- or H+ ions dependent transport).23 The main proteins are TauT (Taurine Transporter) receptors, a high affinity with very low-capacity transporter and PAT1 (Proton-coupled amino acid transporter-1), a low-affinity but high-capacity transporter, able to capturing the circulating amino acid.24 The intraluminal transport of taurine in the kidney is regulated according to the demand of nutrients; in this case, the occurrence of taurine in its soluble form is determinant to exposition of its specific transporters. For the authors, physicochemical properties, as tissue pH and temperature are other important factors for taurine absorption and that the maximum uptake of renal taurine occurs in pH between 7.8-8.0 at 37°C of temperature, which are similar parameters to those found in the final small intestine and initial gut portion of rats and humans.23
Studies of intestinal taurine transporter showed that expression of PAT1 was most abundant in the jejunum, around 1.5-fold higher than elsewhere in the intestine and TauT appears to be similarly distributed, although expression in proximal colon was specially lower than in other regions.24 This can suggest that the lower concentration of the taurine transporters in proximal portion, must remain the amino acid in the intestinal lumen for longer time, exerting a local activity. Finally, there is some basic evidence about taurine can be used as protective for colon cancer, however we must continuous working to consolidate these findings in basic and clinical research.
The research was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) 2019/10746-6 and Programa de Apoio ao Desenvolvimento Cientifico da UNILAGO (PADC/UNILAGO) C.M.C are Conselho Nacional de Desenvolvimento Científico Tecnológico (CNPq) productivity fellows level 2 (CNPq 313435/2019-7). This study was also financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) - Finance Code 001. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Study conception and design: CMC, DHJ, EAG, CRA. Material preparation, data collection and analysis were performed by DHJ, DEC, CBS, CRA. The first draft of the manuscript was written by DHJ, EAG, CMC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data available on request from the authors.
Author declare there are no conflicts of interest.
None.

©2021 Jornada, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.