International Journal of
eISSN: 2381-1803


Review Article Volume 12 Issue 6
NIS Labs, USA
Correspondence: Gitte S Jensen, Research director, NIS Labs, 1437 Esplanade, Klamath Falls, Oregon 97601, USA, Tel (541) 884-0112
Received: October 17, 2019 | Published: November 4, 2019
Citation: Jensen GS, Benson KF. The blood as a diagnostics tool in chronic illness with obscure microbial involvement: A critical review. Int J Complement Alt Med. 2019;12(6):203-212. DOI: 10.15406/ijcam.2019.12.00474
Advanced research into human health and effects of the bacteria around us and inside us is reaching new and unprecedented levels of understanding. However, the information is not reaching the general integrated practitioner fast enough, nor do the findings provide clear feedback to translational medicine for optimal treatment of chronically ill patients. This review paper intends to provide an overview of today’s knowledge, clarify historical observations, and offer some initial levels of scientific grounding for health practitioners. As the studies of biology and medicine grew from infancy through the 19th century, leading scientists identified bacteria as causative agents in many diseases. These findings steered medical microbiology towards the germ theory, the “one-microbe, one-disease” concept. Today, modern microbiology has rocketed beyond such simplified concepts, and shown the capacity of microbial life forms to exchange genetic material, form stealthy biofilm, and live under extreme conditions in forms that are not recognizable as specific species or classical morphological forms.
The scientific understanding and medical use of the human blood for diagnosis and treatment decisions has wavered back and forth over the past 150 years. Early microscopists found morphological evidence for apparent microbial-like forms and associated these with health status and illness. This was followed by a more rigid medical microbiology textbook dogma, based on the concept that the human blood is a sterile environment, and any microbial form observed as a sign of infection, i.e. an invasion by an unwanted microbial form, is specifically linked such that one microbial species will initiate one highly specific pattern of health breakdown.
During the last 20 years, the earlier simplified dogmas have been supplanted. Very recently, it has been shown beyond doubt that broad and complex microbial communities (‘microbiomes’) exist not only in our gut and on our skin, but also in our blood circulation, in cells, tissue, and inside tumors. The intricate interplay between a person’s immune status and health provides a complex backdrop for how the microbial world affects our health and wellbeing.
Keywords: microbiome, microscopy, blood culture, immunoglobulin, oxygen, nutrients, bacteria, cancer, fibromyalgia, chronic pain, inflammation, immune support, preventive health
DNA, deoxyribonucleic acid; PCR, polymerase chain reaction; RBC, red blood cell
Over many decades, practitioners of alternative and complementary medicine have used the study of the living human blood to guide non-mainstream treatment strategies. Schools of thinking developed over time, teaching practitioners an alternative vocabulary to what was being observed by microscopy observations of their patients’ blood. This was at times where mainstream medicine considered the blood a sterile environment, and alternative claims of seeing bacterial and fungal forms were disregarded as ignorant and unscientific.
Today, scientists have definitively proven that human blood contains a wide array of microbial forms, to the point where the term “blood microbiome” is widely used. The most recent developments are happening at a fast pace, so fast that we are struggling with how to integrate the information in terms of practical use in diagnostics and treatment.
Today, we recognize the plasticity of the microbial world of lifeforms. We can look back and appreciate the confusion of early microscopists from a century ago, who did not have our current tools at hand, did not know the nature of genetic material (DNA), and did not have the specific chemical and immunological recognition tools we use today to distinguish pleomorphic microbial forms from normal or stress-related breakdown products of human cells. The classical textbook examples of bacterial rods, coccoid, and spirochete morphological forms is recognized as outdated, as bacterial forms shed cell walls to be able to exchange DNA and to escape immune recognition. Partial and full loss of bacterial cell walls, in combination with the sturdy syncytium colonies recognized as biofilm, in which multiple microbial species may co-exist, has left us with new challenges in terms of detection, diagnosis, and treatment of pathogenic microbial forms.
The review of historical data in context of today's scientific knowledge base presents a challenge. Older data were almost entirely focused on microscope observations, such as the morphology of microbial forms as well as of elements in human blood. The appearance of various shapes and forms was interpreted without the tools to discern human sub-cellular particles from apparent microbiological forms. In retrospect, it seems that some early researchers spent most of their careers observing a mixture of true microbial forms with what we today recognize as normal processes of cellular development and breakdown through apoptosis and necrotic events.1 Rather than ignore or ridicule such older data due to a naïve presentation, we need to acknowledge these early efforts, and bring their essential observations into today’s paradigm.
A foundational example is the conflict and rivalry between Antoine Bechamp and Louis Pasteur. Bechamp argued that ‘granulations’ in biological fluids were elementary units of life, and that unfavorable host and environmental conditions were essential to the destabilization of the health of the host; arguments that have some positive alignment with today’s understanding of nutritional and immunological effects in health and disease. However, Bechamp took his theory to the extreme point of questioning the germ theory and denying that bacteria could invade a host and cause disease. Unfortunately, his standpoint, and the resulting bitter feud with Louis Pasteur, resulted in a loss of respect for Bechamp’s other, more relevant observations. Meanwhile, Pasteur, well known for his contributions to vaccination, microbial fermentation, and pasteurization, received recognition for the disproof of ‘spontaneous generation’ of living forms from apparently non-living organic matter. Unfortunately, this complete rejection of ‘spontaneous generation’ led to a mechanical approach to bacterial biology that dominated mainstream medical microbiology through the following decades.
Robert Koch worked with Pasteur, and built his four postulates in direct extension of Pasteur’s work.2,3 Koch is recognized for the dogma of bacterial causation in infectious diseases that 1) a specific bacterial organism must always be present in all cases of the disease; 2) this bacterial organism must be isolated from the host carrying the illness, and be grown in culture; 3) samples of the bacterial organism must cause the same disease when injected into a healthy animal; and 4) the organism isolated from the inoculated animal must have the same characteristics as the original bacterial organism from the initial host. This paradigm certainly has some value, but it has also limited the scientific thinking and exploration by medical microbiologists for well over a century.4 Today, there are very well documented examples of diseases with bacterial involvement that do not fit any one of these four postulates. Such examples include fibromyalgia and cancer; examples that will be discussed in more depth later in this paper.
Other scientists in the early part of the twentieth century studied the association of microbial forms with cancer, a topic that is hotly revived today. Dr. Oskar Cameron Gruner was a pathologist at McGill University and conducted broad studies of the microscopy of blood elements in cancer.5 Through culture and microscopical observations, he suggested a strong association between tumor environment and the presence of bacterial and fungal forms;6,7 however, he concluded that “the study of the blood is merely an accessory aid to clinical diagnosis”.8 Dr. Gye performed research into the origin of cancer and the etiology of the malignant new growth that discussed the association between microbiology and oncology.9 Several other researchers continued this research well into the 1980s, including Drs. Virginia Livingston-Wheeler and Eleanor Alexander-Jackson, who used vaccine strategies to awaken the immune response to cancers, coupled with diet. Their work did not receive due respect, in part because the researchers hypothesized a linear association between cancer and one specific microbial form. While Gruner’s and Gye’s works were more open and encompassing to possibilities, Livingston-Wheeler hypothesized that one type of bacterium was causative in cancer.10 She named it Progenitor cryptocides and developed vaccine strategies towards it. While Dr. Livingston-Wheeler was criticized for misclassification of the bacterium she was studying as well as a lack of scientific basis for her cancer treatment,11 her theories have been born out on the key role of the immune system in cancer12, the effect of diet including the role of abscisic acid, now shown to be a regulator of inflammation via the PPAR-gamma receptor13 and a role for the inappropriate expression of human chorionic gonadotropin in cancer.14 She was ahead of her time and many of her observations and conclusions have since proved correct. At the time, she did not have the tools to accurately determine the molecular/cellular underpinnings. The use of a vaccine or conditioning of the immune system to respond to cancer is the basis for the cutting-edge cancer immunotherapies currently being developed.15
To complete this historical review, several other researchers must be mentioned, since their work has caused confusion amongst patients seeking cures for various illnesses. These researchers have disregarded important cornerstones of accepted mainstream biology, pathology, and hematology, and created alternative pockets of knowledge. They performed elaborate work over decades, but their conclusions were not successfully incorporated into today’s scientific platform for how we currently think about living organisms. Some of these researchers (Table 1) claimed to have observed and isolated small living particles that were associated with provision of energy to higher living organisms, which remains hypothetical. These researchers all associated microscopy observations of human blood with the state of health, in similar manners as did the researchers described in Table 2, but they developed their own alternative descriptive vocabulary of their observations. This was a fundamental reason for the rejection of their core hypotheses by mainstream science. A detailed description of their alternative vocabulary and biological concepts is not the scope of this paper. Whether any of the observations of very small dense particles in the blood overlap with modern discoveries of Very Small Embryonic Like stem cells is unknown. Whether in the future we will prove alternative energy generation mechanisms, associated with particles in the blood, is an open question.
Researcher |
Year |
Particle name | Hypothetical role |
Bechamp A |
1816-1908 |
Microzyme |
Symbiont that could turn |
Reich W |
1897-1957 |
Orgone |
Carrier of energy |
Enderlein G |
1872-1968 |
Endobiont |
Symbiont that could turn |
Naessens G |
1924-today |
Somatid |
Symbiont that could turn pathogenic, carrier of energy |
Table 1 Alternative biologists and their hypothetical models for blood particles
Scientist |
Year | Scientific contributions |
|
||
Pasteur L |
1846-1895 |
|
Disproving spontaneous generations, Pasteurization, Vaccination. |
||
Koch R |
1866-1910 | Koch's four postulates: Cause and effect association |
|||
Gruner O |
1877-1972 |
|
Hematology, bacterial and fungal association with tumors. |
||
Gye WE |
1884-1952 |
|
Bacterial association with tumors. |
||
Livingston-Wheeler V |
1906-1990 |
Vaccination to awaken immune response to tumors | |||
Mattman L |
1912-2008 |
|
Comprehensive documentation of bacterial pleomorphism. |
Table 2 Early scientists who contributed to the recognition of microbial forms in health and disease
Some of the alternative biology researchers were successful in outlining treatments for cancer and other illnesses; however, they explained their rationale for their treatment strategies by the alternative biological concepts they believed in. Despite the misleading scientific vocabulary surrounding these researchers’ rationales for their treatments, two of the researchers developed remedies that may be explained in today’s scientific language.
Enderlein developed a series of microbial-based highly dilute remedies based on his theories. Reviewing his remedies today, other mainstream explanations for the mechanisms by which these remedies act include immune activation and regulation. Modern versions of such remedies thrive today in German biological medicine, where one of the remedies based on fungal antigens has been researched using modern proteomics and metabolomics testing, showing selective activation of components of the immune system.16 The remedies available today are designed to present various bacterial and fungal antigens to the immune system, which overlap with Livingston-Wheeler’s vaccines, as are the vaccine strategies being developed today to help alleviate chronic pain and cognitive dysfunction symptoms via vaccination with bacterial toxins.
Naessens’ remedy 714X, a dilute blend of salts and camphor injected into lymph nodes, typically in the groin, may also have acted directly on the immune system. Lymph nodes are pivotal microenvironments for antigen presentation and decisions on cellular responsiveness versus an unresponsive state called ‘immunological anergy’. Camphor is an active compound in many botanical essential oils and has been shown to increase cell viability of some types of immune cells.17 If 714X is to gain a more accepted role in immune support, much additional research is necessary to fully understand the signaling and reprogramming of human immune cells after a pulsed exposure to 714X.
In conclusion to the historical perspective and our current knowledge, it is not always a simple process to discern living and non-living particles. Both types of particles can derail a person’s health status. And, how do we define ‘living’? Our current definition of a living form is debatable,18 and our current definition of life may one day be as over-simplified and outdated as Koch's four postulates are today. That said, most scientific teams currently agree to the following definition:19
This definition encompasses all known bacteria, fungi, single-celled animals, and higher plants and animals. However, there are important exceptions: 1) Some cell types shed DNA (such as mammalian mature red blood cells), and the mature and metabolically active red blood cells are considered living, until they undergo an orchestrated death process known as erythroptosis. 2) Some fully differentiated tissue cells are no longer capable of replicating; however, they are certainly considered living cells.
On the verge of this definition of living organisms, we find numerous particles isolated from blood, body fluids, and tissue, discussed in the scientific literature in recent years. These particles fit some, but not all, of our current criteria for defining living forms, and are not presently considered alive, even though they are intricately involved in human health and disease (Table 3).
Particle name |
Known function |
Viruses |
Parasitic particles containing genetic material, able to hijack the function of host cells to produce more viruses. |
Prions |
Infectious disease-causing particles made of proteins. Prions are neither bacterial nor fungal nor viral and they contain no genetic material. |
Nanobacteria |
Self-replicating calcifying particles that can, in some cases, contain genetic material such as viruses.21–23 |
Metal clusters |
Proteons with homology to the hemoglobin-alpha chain, folded around metal clusters, |
Artificial life |
Generation of single-cell life forms from building blocks, and addition of man-made synthetic DNA.26,27 |
Table 3 Very small particles on the verge of life
In addition, elaborate research is being performed regarding biological self-organization, including the synthetic generation of cell-like structures in the absence of defined living forms. Examples include the generation of self-replicating cellular structures from nutrient soups bombarded with energy through UV radiation, lightning etc. Some of this research, however, has borrowed genes from existing bacteria, to generate a minimalistic cell for industrial purposes.20
The human microbiome
Today, we recognize the immense crosstalk between bacterial and higher life forms. As we continue to document the vast impact of microbial species on maintenance of human health, the term ‘microbiome’ has been coined to describe the world of microbial forms associated with the human body. Most of these microbial forms are, technically speaking, on the exterior of the human body, including the skin, oral cavity, and gastrointestinal system. However, very recently, it has been shown that the human blood and tissues also contain multiple microbial forms in living, actively metabolic states. It is well-documented that the microbial community, “microbiome”, on skin, in the oral cavity, and in the gut, has an immense effect on the health of the human host.28-31 An integral collaboration exists between microbial forms colonizing our gut, and our immune function, metabolism, and brain function.32 This mutualistic relationship includes the breakdown of complex carbohydrates, production of vitamins and immune-modulating metabolites and protection against pathogens.
Recent research has verified that the blood, once thought to be a sterile environment, is in fact colonized by a microbiome of its own.33,34 It is very likely that the microbial forms in the blood stream predominantly enter the blood via the gut mucosal barrier,35 and the oral cavity.36 It is an inherent property of the immune system along our mucosal surfaces to continually sample the microbiome, and maintain an alert immune response to potential pathogens. This sampling includes cellular uptake (phagocytosis), which may or may not lead to the ultimate destruction of the microbe. If complete destruction of the microbial form does not take place inside a phagocyte, the microbe may escape and transiently exist as a free form in blood or tissue, likely to be detected and destructed by other immune defense mechanisms such as, for example, antibody-mediated immunity. Alternatively, the phagocyte may not successfully destroy the microbe, and the microbe may set up residence inside the phagocytic host cell, and modulate the host cell metabolism, such as Ehrlichia subspecies.37,38 Also, the red blood cell is increasingly recognized as a location for microbial forms, a topic that we will discuss in more detail below.
The medical term ‘sepsis’ refers to obvious microbial invasion in the blood stream. Sepsis is indisputably a life-threatening medical emergency, and the diagnosis and treatment of sepsis is not the scope of this paper. Our aim is to review and discuss less obvious microbial presence and discuss the assessment of pathogenic potential of such microbes.
In the absence of acute illness, microbial forms can be seen in small amounts in the human blood. In addition, it is becoming increasingly clear that microbial forms colonize tissue and contribute to local and systemic imbalances in nutritional status and immune function. Obscure microbes are key contributors to chronic illnesses and may serve as vectors/carriers for pathogenic DNA, that, when expressed, may undermine the host biology. However, it is important to note that there is not a linear association between a specific microbial species and a particular disease. Certain microbes have an affinity for specific tissue. For example, Propiniobacterium acne, known as one of the bacterial species contributing to chronic acne in the skin, also has an affinity for the prostate, and has been found in prostate tumors in humans and animals.39,40 This will be further discussed in the examples below.
Once an obscure microbial presence is established in the human host, we do not have clear and definitive tools to grade to what extent it is potentially harmful to the host. The definition of a pathogen has changed dramatically over the past 130 years. A non-pathogenic bacterium may serve as a vector for pathogenic viruses. Each pathogenic mechanism of action opens a door to treatment, as discussed later in this paper, where we discuss a comprehensive integrated strategy, including anti-microbial herbs and electrostimulation, immune modulation, reduction of inflammation, detoxification, and gut health.
Microbial entry can happen via introduction across mucosal membranes, and across the skin barrier, and ideally leads to immune recognition and elimination. However, harmless and harmful microbes alike may escape and establish colonies in areas where they shield themselves from further immune recognition. From a diagnostic viewpoint, the challenge is to dissociate the presence of a non-pathogenic microbial form, destined to be destroyed by normal immune defense mechanism, from an obscure pathogenic microbial form escaping from immune recognition in multiple ways that makes the microbial form very difficult to detect by most methods.
The human blood is easy to obtain for diagnostic purposes and is in most cases appropriate and sufficient. Basic observation of microbial organisms in human blood samples using microscopy works in some cases for crude detection of classical forms, such as spirochetes in syphilis and acute Borreliosis (the early stage of Lyme’s disease). However, there are situations where the traditional medical microbiology methods are misleading or insufficient. The testing of either freshly drawn blood samples or cultured blood using microscopy, serological, biochemical, or molecular biology methods have limitations and may give negative results, even in cases of highly active illness due to bacterial presence (Table 4). Recently, immune response-based testing methods allow for evaluation of active ongoing immune reactivity to specific pathogen antigens.41,42 Often, integrative health practitioners choose a select panel of tests, combining an effort to demonstrate bacterial presence and identifying an ongoing immune activity towards it.
Method |
Limitations |
Blood microscopy |
Morphology alone does not identify microbial forms, and without staining does not distinguish between breakdown products from human cells and microbes. |
Blood cultures, optical density |
Non-specific documentation of microbial presence in a blood sample through increased turbidity over time in culture. |
Serology (Antibodies) |
Measurement of immunoglobulin produced by the host organism towards specific molecules from a microbe. Indirect proof that the patient’s immune system has at one point produced a response to a given microbe. The test results show that the host immune system has responded to the microbial form but does not show whether the microbe is still present. |
Blood PCR Molecular genetics |
If a sufficient quantity of bacterial DNA is present in a form that is recognized and amplified by the PCR method used, then a signal will indicate the presence of bacterial DNA but not necessarily confirm the presence of live bacteria. |
Blood cultures followed by PCR |
The intent is to increase successful detection of a microbial form by PCR, by first allowing the microbes to grow in culture, so it is more likely that the PCR test will produce a positive signal. Limitations include whether a microbe will grow under the culture conditions used, and whether the PCR amplification method is suitable. |
Immune cell cultures (EliSpot) |
Measurement of T cell secretion of Interferon-gamma when challenged with bacterial antigens. |
Biochemistry/Metabolomics |
Provide evidence for pathogenic activity and disturbance of the host environment. This may include detection of toxins or other secreted bacterial products. This has promise for future test methods, in cases where a microbial form may be present in active or inactive forms or have donated pathogenic genes to other microbes. |
Table 4 Methods for detection of microbial forms in human blood samples
There is often a contradiction between microscopy observations and molecular genetics examining the presence of nucleic acid. Seemingly living forms can be observed in fresh blood or blood cultures by microscopy, seeming to move with purpose, multiplying over time in culture, and not fitting well-defined morphological forms of apoptotic bodies. These forms include those derived from human host cells, platelets undergoing systematic disintegration (blebbing) as part of their normal adhesive and secretory activities,43 or other natural breakdown processes of human cells and sub-cellular elements. However, the success versus failure of detecting DNA from such samples is not trivial (see the section ‘Example 1 – Fibromyalgia’ below). It is also important to keep in mind that forms observed by microscopy may represent more than one microbial species, and the success of detecting DNA from one species does not preclude the co-existence of other species; however, this introduces a bias in making cause-and-effect conclusions.
Furthermore, some microbial forms (or their potentially pathogenic genes) can be invisible to most diagnostic tools while still contributing significantly to severe chronic health problems. A microbe that is contributing to a health problem may no longer be in the blood circulation or may never have been in the blood (such as P. acne that may enter the prostate from the skin surface via the ureter). Specific microbes have affinity for certain environments. Examples include:
Twenty years ago, the pleomorphic nature of bacteria was still highly controversial and hotly debated.45 Today, there is no remaining doubt that most if not all bacteria can transition between classical free-living “planktonic” forms with cell walls, cell-wall deficient L-forms, cell-wall depleted protoplasts, metabolically quiescent ‘persister’ cells, and biofilm. Cell wall-depleted protoplasts are widely used in genetic engineering, since the lack of cell wall allows DNA transfer. Biofilm is a group of microorganisms that adhere to each other, typically on a surface, and are embedded in a protective matrix of extracellular polymers. Biofilm may be composed of a single microbial organism, or may be a community of many different species, cohabiting in the same polymeric matrix. Within biofilm, bacteria can live as protoplasts, and save the energy expenditure of producing cell walls. This further allows for genetic transfer between organisms embedded in biofilm, rendering this a highly active microbial breeding ground for new traits, as well as the exchange of genes allowing for antibiotic resistance.
Biofilm involving pathogenic bacteria poses extreme challenges for the sterilization of hospital equipment and are responsible for multiple nosocomial infections.46 Biofilm on dental surfaces is involved in dental decay,47 and much recent research has been devoted to find ways to inhibit the metabolic activity in dental biofilm. In addition, there is an increased recognition regarding biofilm involvement in chronic or recurring illnesses of other mucosal surfaces such as lung and bladder, including cystic fibrosis, recurrent bladder infections, and systemic lupus erythematosus development.48 More controversial is the involvement of biofilm inside the blood and tissue of chronically ill patients, including Post-Treatment Lyme Disease Syndrome. It was shown in a mouse model of Borrelia burgdorferi-induced arthritis that biofilm-microcolonies of stationary phase ‘persister’ cells caused more severe illness symptoms.49 Several biofilm-forming and intracellular pathogens can coexist in patients with this illness, and diagnosis and treatment pose extreme challenges. Most antibiotics do not penetrate biofilm. Some antibiotics may be able to arrest growth and metabolic activity in free-living forms of a bacterium but may also trigger evasive tactics by the microbe to form biofilm and to enter a quiescent state as ‘persister’ cells.50 Multifaceted natural treatments combined with immune support has shown promise, and herbal extracts, many used in traditional folk medicine, are showing promise in inhibiting biofilm formation.51–55 Novel treatments using bee venom as immunotherapy56 has also shown promise in clinical trials in cancer patients,57 arthritis,58 and other illnesses, and has been shown to disrupt Borrelia biofilm in vitro.59 The current theory is that such treatments help support the patient’s immune system, while also altering the gene expression profile of the biofilm-forming bacteria so they are no longer able to remain in an obscure form, but must emerge as free forms susceptible to treatment (Figure 1).
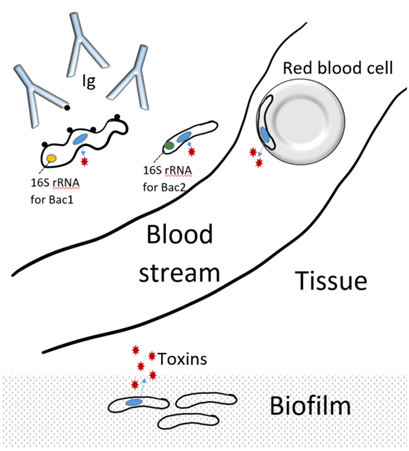
Figure 1 Diagram showing two types of bacteria (Bac1 and Bac2).
Bac1 carries a gene for toxin production (blue loop), and donates that gene to Bac2. Bac1 carries the gene for 16S ribosomal RNA (rRNA) for its species, whereas Bac2 carries the 16S rRNA for its species. Detection by PCR would show Bac2 in red blood cells and biofilm, but would not detect the original invader, Bac1. The immune system developed antibodies (Ig) towards cell wall components (Black dots) on Bac1, but are not detecting the cell wall deficient Bac2, neither in tissue, inside red blood cells, nor in biofilm.
Bacteria have been found inside human red blood cells in healthy60,61 and chronically ill people.62,63 The inner membrane of the red blood cell may provide a matrix for biofilm generation where multiple pleomorphic microbial forms could co-exist, as part of a dynamic biological diversity with pathogenic potential. Our work on intracellular pleomorphic forms has focused on obscure forms in erythrocytes. Our findings indicated a possible role for proteobacteria in taking up an obscure residence in erythrocytes and possibly acting as a carrier of potentially pathogenic bacterial genes as well as viral elements. We suggest that these genes expressed in intraerythrocytic microbial forms may possibly represent a way for pathogenic elements to be obscurely expressed, while remaining unrecognized by current diagnostics. The complexity of microbial infiltration in the human body may contribute to an increased stress load on the immune system.
A recent study on the phylogenic profile of the blood microbiome determined that the majority of bacterial DNA found in blood fractions is from the Proteobacterium phylum and that there are differences in bacterial profiles of different blood fractions that include an enrichment for certain pathogen taxa in red blood cells.31 mature mammalian red blood cells (RBC) are practical research models for these reasons: They do not contain autologous DNA, nor do they contain mitochondrial DNA. The association of pleomorphic microbial-like forms with red blood cells is therefore easier to interpret, both by microscopy and by molecular genetics methods.
Patients suffering from fibromyalgia and other chronic pain-associated syndromes, are seeking treatment for chronic pain not caused by physical trauma, and as in many other chronic illnesses, the diagnostic process is in shambles, where the principal guide is the clinical symptoms. With all our advances, treatment is predominantly based on clinical symptoms. Clinical test results are more being used to eliminate known illnesses and organ dysfunctions, rather than to positively support a treatment strategy. Current treatment modalities include pain management either directly or by an improved sleep quality and circadian rhythm. The association with poor sleep and deregulated circadian rhythm points to an immune dysregulation, due to the intrinsic association between circadian rhythm and immune surveillance.
The red blood cell offers a unique microenvironment for microbes to hide from immune recognition. It has been suggested that triboelectric effects of the red blood cell membrane, generated through friction in the circulation, can attract bacteria.64 In studies of the blood microbiome, it has recently been shown that red blood cells contain more potentially pathogenic bacteria than other fractions of the whole human blood.65–67 We established protocols67 using improved methods for dealing with a) low copies of unknown bacterial DNA, and b) extraction of DNA from difficult sources such as soil and human blood, where acids as well as minerals such as iron create a challenge for more conventional DNA extraction methods.
Our research team has evaluated the presence of pleomorphic microbial forms associated with erythrocytes (red blood cells) in patients with chronic pain. We found that, despite negative cultures from medical diagnostics laboratories, we could culture microbial forms, either by culturing the whole blood, or the purified red blood cells. A more robust outgrowth of bacterial forms was seen when serum was removed, and when the oxygen pressure was reduced.68 The appearance of microbial forms in red blood cell cultures was highly influenced by nutrients in the culture medium, suggesting that nutrition-based interventions may assist the patient in controlling the microbial burden. Furthermore, addition of herbal extracts to the cultures could suppress the microbial growth manifold. An example of intraerythrocytic DNA, stained with a green fluorescent probe, is shown in Figure 2.
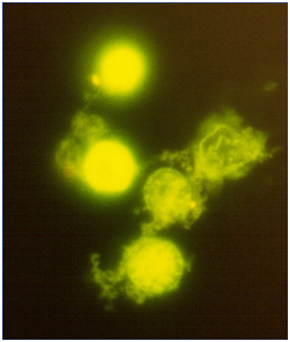
Figure 2 Fluorescence microscopy images of red blood cells from a patient with fibromyalgia.
The red blood cells were cultured in the absence of the patient’s serum, to allow the microbial forms to grow. The green fluorescent DNA stain Syto9 was used to demonstrate the presence of bacterial forms inside the red blood cells, and after prolonged culture also emerging free bacterial forms.
In a recent study, focused on fibromyalgia patients in Oregon, USA, we showed association of microbial forms with red blood cells, where 16S rDNA analysis documented the presence of bacterial DNA from the genus Aquabacterium.67 Using sensitive molecular genetics methods; we amplified bacterial DNA from 7 out of 8 fibromyalgia patients, and 3 out of 4 healthy people. There was not a clear association between positive cultures and positive DNA amplification, since some cultures appeared to have microbial forms, but the DNA amplification did not successfully detect bacterial DNA using a 16S rDNA universal primer. The high degree of variability suggests that multiple microbial forms may coexist in the red blood cells, where some are not culturable, but may still be actively interfering with host biology, and the severity of the obscure presence of bacteria may interfere with host physiology (Table 5).
Reducing oxygen saturation by inhabiting the interior of red blood cells. |
Reducing oxygen delivery to tissue due to reduced red blood cell flexibility by biofilm formation along the interior of the red blood cell membrane. |
Competing for nutrients including minerals and trace minerals. |
Secreting hormone-like compounds, interfering with host metabolism. |
Secreting toxins affecting the immune and nerve system of the host. |
Contribute to inflammation and oxidative stress. |
Serving as a host for pathogenic bacteria and/or viral elements, thus contributing to a highly variable and inconsistent presentation of clinical symptoms. |
Trigger prolonged auto-immune disease, even if the bacterium subsequently leaves the system. |
Table 5 Potential interference by intra-erythroid bacteria with host immune system in fibromyalgia
Previous publications from various research teams associating viral elements or mycoplasma DNA with fibromyalgia and chronic fatigue syndrome may need to be reviewed considering the possibility that segments of viral and/or mycoplasma DNA was carried by proteobacteria in those patients. To further question whether the microbial presence had the potential to contribute to inflammation, the bacteria from red blood cell cultures could secrete metabolites capable of interacting with the human immune system. We hypothesize that the bacterial secretion of metabolites is controlled in the fibromyalgia patient, but during flare-ups could be a contributor to inflammation and pain, and central nervous system dysfunction.
Associations suggesting that fibromyalgia is caused by non-celiac gluten insensitivity is inconclusive, and simply points to a delicate gut system and inflammatory conditions being associated but not causative of the disease. Interestingly, the stimulation of the patient’s immune system using vaccination with bacterial toxins has shown some success in reducing pain and other symptoms in fibromyalgia patients. The recent publication by Zachrisson’s team used Staphylococcal toxins for vaccination and documented an association between the antibody level and clinical improvement.69 The antibodies were specifically targeted at bacterial toxins and enzymes. The antibody response to cell wall components was less impressive, which is not surprising if bacteria in fibromyalgia patients are predominantly cell-wall deficient, residing inside red blood cells, or residing in the gut. This suggests that immunological anergy (unresponsiveness) may be present before the vaccination program, and may be overcome, thus awakening the immune awareness not only to Staphylococcus but also other bacterial forms. We suggest that this strategy is an extension of Livingston-Wheeler’s treatment strategies.70
Example 2 – Cancer
The historical observations of associations of bacteria with tumors, originally observed by Gruner, Gye, and Livingston-Wheeler,5,9,10 is now a fact that opens the door for using this therapeutically in several ways. Over the past few years, it has been well documented that bacteria will home to tumors, and migrate into the tumor tissue, rather than seek healthy tissue. This property has been exploited for the development of engineered bacteria for use in visualization of tumors and metastases in animals.71,72 For cancer therapy, tumor-seeking bacteria have been designed to carry compounds that kill tumor cells and attract immune recognition.73 This has enormous medical potential in many applications where tumors may be inoperable, or surgery carry a very high risk of complications, such as, for example, brain and prostate.
Our exploratory research aimed to verify the presence of bacteria in tumor tissue, using both our culture method (see Fibromyalgia section above), and conventional agar plates. We used a combined amplification strategy, where we first attempted to expand the numbers of bacteria through a period of culture, then followed by amplification of DNA using our sensitive 16S rDNA PCR method. Among three tumors from two different animals we identified four different species (Table 6). Two were propagated in liquid culture and two by conventional chocolate agar plates. There was no overlap between the species detected in liquid culture versus agar, suggesting that the liquid culture method may allow detection of additional species, and expand our knowledge of the co-existence of multiple bacterial forms within the same tumor. Aligned with recent literature on prostate tumors, we detected P. acne in a canine tumor of the testes, but we found that it was accompanied by at least two other bacterial species. Also, even when using both culture methods in parallel, combined with our sensitive PCR, we did not detect bacteria in all tumors; at this point we speculate that bacteria may have been present but at numbers too low to amplify with the combined culture/PCR method (Figure 3).
Sample |
Tumor |
Liquid Culture |
Chocolate Agar |
Bacterial Identity |
Dog 1 |
Liver |
+ |
- |
Pseudomonas fluorescens |
Spleen |
- |
- |
|
|
Dog 2
|
Testes |
+(1) |
+(2)a +(3) b |
1. Unculturable bacterium |
|
2. Bacterium Te66A |
|||
3. Propionibacterium acnes |
||||
Table 6 Results of DNA Sequencing of 16S rDNA PCR products recovered from canine tumor material
aLarge flat greenish colonies on chocolate agar plate, bacteria had coccus type morphology.
bSmall reddish colonies on chocolate agar plate, bacteria had bacillus type morphology.
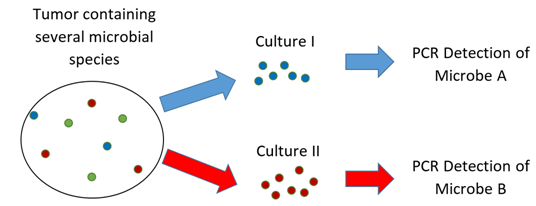
Figure 3 Diagram showing a tumor containing three types of bacteria.
The amount of bacterial DNA in the tumor tissue is low, and culturing was used to help amplify the copies of DNA before PCR amplification. One type of bacteria could multiply in culture medium I, whereas the other bacterium preferred growing in culture medium II. None of the culture conditions supported expansion of the third type of bacterium in this example. If the tumor tissue had not been cultured, the PCR may not have detected any bacteria. If only one culture method had been used, the other bacterium would have remained undetected. The presence of unculturable bacteria remains possible, present at levels below levels of detection.
Keeping in mind the current insufficient routine diagnostic techniques, we must re-think how to define microbial presence, infection, and disease. Much attention should focus on low-grade chronic infection and inflammatory processes, which can be fertile grounds for malignant transformation and tumor development and progression. It is necessary to revise the causation of many diseases, including cancer, to include microbial factors in the disease process. On a positive note, this opens additional windows of opportunity for prevention and therapy. With cancer reaching epidemic proportions, it is cheaper for society to increase the broad use of anti-inflammatory agents such as antioxidants and nutritional support for all citizens, than to finance surgery, radiation, chemotherapy, immunotherapy, or custom-designed anti-cancer vaccines for up to 40% of its citizens. This is not to ignore the genetic predisposition for cancer, but to add to this picture by acknowledging the preventive or life-extending measures of optimizing the immune system and reducing chronic inflammatory conditions.
To what extent the bacterial presence in tumor tissue may have implications on tumor development and progression is a separate question. It was shown that injection of P. acne bacteria into rat prostates reproduced similar inflammatory processes seen in prostatic hyperplasia.35 The association of bacteria with tumors confounds the cause and effect conclusions of some chemotherapeutic agents. For example, the commonly prescribed chemotherapy agent doxorubicin is an anthracycline antibiotic. While acting as a chemotherapeutic through its action as a DNA intercalating agent with broad effects on DNA replication and transcription in many tumor types, some of its effectiveness could be due to its antibiotic properties, particularly at the level of the mitochondria.74
As our knowledge of the complexity of human/microbe interactions increases, so does our appreciation of the specific strategies applicable to supporting human health. The goal is to reduce the advantage of potentially pathogenic microbes, while providing an environment of optimal advantage for the body's own cells. Supporting a strong immune system and gut health, reducing inflammation, while maintaining a high nutritional status, is pivotal to all the examples we have discussed here.
Long-term, integrated, multi-faceted strategies are crucial. Single treatment modalities are important, but often only offer short-term relief. For example, if a tumor is large and obstructive, surgery and other mainstream treatments are lifesaving, but do not help change the immunological anergy that allowed the tumor to develop in the first place. In acute infections, antibiotics can be lifesaving, but may not address obscure pockets of surviving biofilm of the pathogen. In many chronic pain patients, who are often depressed, mainstream or alternative methods of pain reduction and mood elevation are a first line of support to help the patient get to a state where they are motivated and capable of embarking on a comprehensive treatment strategy. However, none of the single treatments are optimal as stand-alone solutions. Also, alternative treatments such as sound and electrical frequencies, as well as the anti-inflammatory effects of Earthing,75,76 are claimed to be successful, and urgently need to be subject to systematic clinical studies, using rigorous study designs. The goal is to create a logical and science-based framework for optimal integrated treatment. Searching for optimal treatment protocols for chronic illnesses associated with obscure microbial forms should be based on the many preliminary evidences for ways to favor the immune system while disfavoring the microbial organism metabolism (Table 7).
Method |
Conventional (mainstream) medicine |
Complementary medicine and self-help |
Oxygen |
Hyperbaric oxygen |
Breathing, yoga |
Nutrition |
Dietary recommendations |
Vitamins, minerals, herbs |
Immune support |
Pharmaceutical immune modulation |
Vitamins, minerals, herbs, colostrum, medicinal mushrooms |
Circadian rhythm |
Sleep medication |
Melatonin, herbs, healthy sleep cycle in dark |
Antimicrobial |
Antibiotics |
Herbs, oxygen, natural antimicrobial toxins (ex: bee venom) |
Anti-inflammation |
Medications such as NSAIDS, prednisone |
Herbs, diet, probiotics for gut health |
Gut health |
Antacids, laxatives |
Probiotics, prebiotics, digestive enzymes |
Detoxification |
Hydration |
Support of gut, liver, kidney, skin function |
Brain/body communication |
Not a focus for treating illness. |
|
Neurological health |
Anti-depressants, tranquilizers |
Diet, vitamins, minerals, herbs |
Table 7 Summary of integrated strategies in prevention and therapy
Microbial forms exist in human blood and tissue and interact with the human host. In most cases, the human host controls the growth and metabolism of the bacteria. However, in some cases, the bacterial forms are actively interfering with the host biology and health. Antibiotics may help in acute and potentially life-threatening situations, but are not curative, as they may not reach cell wall-deficient protoplastic forms, or forms living in biofilms. Multi-faceted treatment strategies are necessary to regain control of health, in part through antibacterial treatment, and in part through support of the person’s immune system, hormonal balance, and nervous system.
With the increased technical ability to detect large arrays of microbial forms in human blood, the pressing question is how to use this information in modern medicine. Our ability to use the information in a meaningful diagnostic framework is lagging but could improve rapidly with systematic studies across many types of patient populations. The study of proteomics is needed and should not be limited to searching for microbial toxins and metabolic markers, but should encompass simultaneous assessment of the immune, nutritional, inflammatory, and neurological status of the human host. The study of the metabolic activity of obscure microbial forms is urgently needed, in context of mainstream antibiotics versus treatments with combinations of natural antimicrobial compounds from herbs, mushrooms, bee venom, and clays. With this, we may truly be at the doorstep to a very powerful implementation of ‘personalized medicine’, where the treatment of ‘dis-ease’ may move away from traditional diagnostic terms, into the practice of truly modern, science-based, integrated, and holistic medicine.
The research was conducted at NIS Labs. The work was sponsored (in part) by the Fetzer Franklin Fund of the John E. Fetzer Memorial Trust. Parts of the early pilot work was performed at McGill University, and sponsored by the Alberta Cancer Research Foundation. Additional support was received from Michael Sheehan at Bioresource Inc., and a private donation from Mr. Al Schaefer. We thank our colleagues Dr. Ali Khamessan, Patricia Huerta, and Christian Drapeau.
The authors have no financial interest in the subject matter.

©2019 Jensen, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.