International Journal of
eISSN: 2381-1803


Review Article Volume 6 Issue 2
Herbal Analysis Services UK & Pharmacognosy Research Laboratories, University of Greenwich, UK
Correspondence: Solomon Habtemariam, Herbal Analysis Services UK & Pharmacognosy Research Laboratories, University of Greenwich, Chatham-Maritime, Kent ME4 4TB, UK, Tel +44-208-331-8302
Received: February 16, 2017 | Published: March 30, 2017
Citation: Habtemariam S (2017) Going Back to the Good Old Days: The Merit of Crude Plant Drug Mixtures in the 21 st Century. Int J Complement Alt Med 6(2): 00182. DOI: 10.15406/ijcam.2017.06.00182
Throughout human history, natural products have been effectively employed as sources of medicine both in their crude mixture forms and as purified single chemical entities. With advances in medicinal chemistry researches and availability of drugs from synthetic sources, the use of natural medicines in the last few decades have been greatly undermined in the developed world and those that survived must have to fit for purpose through rigorous quality control checks and/or evidence of quality, safety and efficacy. This article outlines the merits of natural medicines for the 21st century complex diseases through various concepts of therapeutic approaches. The high prevalence of more complex diseases that require approaches of polypharmacology principles among others are discussed.
Keywords:plant medicines, herbal medicines, drug targets, dirty drug, smart drug, bioassay-guided-isolation, synergism, polypharmacology, multifunctional compounds, complex diseases
Pharmacology itself has been written and shaped up by natural drugs
Can one imagine pharmacology as a subject and as we know it today without the contribution of natural products to its development? Let us use neuropharmacology as an example and scrutinise the contribution of natural products to our understanding of neurotransmission, receptors and/or ion channels. The black widow spider venom stimulates the release of neurotransmitters release from packed vesicles in nerve ending leading to vesicles depletion and neuromuscular transmission blockade. Likewise, Botulinum toxins from Clostridium botulinum are among the best known neurotoxins that block the release of the neurotransmitter acetylcholine from the nerve ending leading to muscle paralysis. On the other hand, tetrodotoxin from pufferfish and other sources and saxitoxin from shellfish (algae and cyanobacteria origin) are potent inhibitors of sodium channels in nerve cell membranes and hence inhibit nerve conduction across the axons. The observation of effects of such toxins in biological systems has helped us to understand the very mechanism of communication between two neurons and/or neurons and muscle cells. At the receptor level, the various snake venoms/toxins such as α-Bungarotoxin that selectively bind to some receptor populations have led to the classification of the various receptor types. The classification of acetylcholine receptors as muscarinic, based on the agonistic nature of muscarine isolated from the mushroom Amanita muscaria; or nicotinic acetylcholine receptors named due to the agonistic nature of nicotine in these receptors are further good examples of pharmacology shaped up by natural products. The story of the muscle relaxants development through knowledge of curare poison that people used for centuries but latter characterised to give the active principle tubocurarine also served as the backbone of pharmacology development as a subject in this area. Hence, pharmacology in essence was defined and developed as a subject by drugs and toxins from natural sources. Intriguingly, natural medicines that served as the backbone of pharmacology have been subjected to modernisation in the last century through demonstration of scientific evidences that came from pharmacological studies. Hence, making plant/herbal medicines to fit for purpose in the 21st century also require the close scrutiny of traditional medicinal claims through evidence(s) from pharmacological studies.
The drive for a pure single chemical entity as a drug
One of the great achievements of the last century has been in the identification and utilisation of single chemical entities, instead of crude mixtures, as drugs. The concept of one purified drug against a specific target and disease has been the principal goal of modern drug development. This approach highlights the therapeutic concept of one drug→one target→one disease.The discovery and utilisation of antibiotics right after the Second World War is the classical example of how this concept helped the human race to win the various battles against microbial pathogens. The penicillins targeting the bacterial cell wall synthesis machinery, which is unique to the pathogen, offered a selective therapy that could be employed in the human host with little side effect(s). While the war against microbes will continue to be fought for centuries to come, the contribution of such an approach and its therapeutic implications for many other disease conditions has been given central role in modern drug therapies. On this basis, pharmacology in combination with modern phytochemicals methodologies has been employed to characterise the active principles responsible for the claimed medicinal properties of herbal medicines. Pharmacology often in combination with phytochemistry also became the accepted norm to provide the evidence of quality-safety-efficacy of natural medicines. The quest for evidence in this direction was further developed as a regulatory necessity both at national, reginal and international level; good example is the European Union legislations on herbal medicines use. Undoubtedly, going through such systematic scrutiny of plant medicines offer the opportunity to identify novel drug candidate that may be used as a single chemical entity. On the other hand, studies on the pharmacology and phytochemistry of plant medicines allow standardisation of herbal medicines based on the identified active principle(s).
To date, many herbal medicines in Europe are being sold with quality assurance directed with known active principle(s) for a well-defined pharmacology related to the claimed medicinal uses. Accordingly, researches in our laboratories in the last three decades have also been directed in the characterisations of hundreds of medicinal plants collected from almost all corners of the world. One classical example from the European medicinal plants was that of gravel root (Eupatorium purpureum)which is extensively used to treat chronic inflammatory diseases including rheumatoid arthritis. By using in vitro and in vivo models of anti-inflammatory assays, the active principle has been isolated and characterised as a benzofuranderivative,1 (Figure 1).1-3Through similar approaches, the anti-haemorrhagic use of Hamamelis virginiana.4 and anti-inflammatory effect ofAndrographis paniculata.5, (Figure 1) were among the countless examples where we have provided scientific evidences to substantiate traditional medicinal claims (see our extensive publications at http://www.herbalanalysis.co.uk/publications-new.html ). The merit of science as a pillar for making such medicines fit for the current purpose must therefore be favourably viewed by all stakeholders: the consumers, the pharmaceutical/herbal industry and the scientific community.
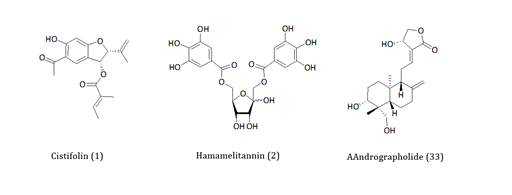
Figure 1 Examples of pharmacologically active principles isolated from herbal medicines. The structures of antiinflammatory compounds from gravel root or Eupatorium purpureum (1), Hamamelis virginiana (2) and Andrographis paniculata (3) are shown.
Smart drugs for complex diseases
Around 795 million people in the world (one in nine people) today do not have enough food to lead a healthy active life .6. This is one of by far the most depressing figures of the 21st century we are living in but the prevalence of undernourished people in the last decade even in developing countries, were the vast majority of hungry people live, has reduced by 42 percent .7. In contrast, more than 1.9 billion adults (18 years and older) were considered overweight in 2014 of which over 600 million were obese.8 This means that most of the world's population today live in countries where overweight and obesity kills more people than underweight; or where excess kills more than deprivation. The growing obesity figure mirrors its major associated disease, diabetes, with global estimate of 422 million in 2014 and its prevalence risen from 4.7% in 1980 to 8.5% in 2014.9 The other distressing figure where obesity/diabetes play major role came from the cardiovascular disease, a number one killer, registering about 17.5 million people death each year and an estimated 31% of all deaths worldwide.10 Raised blood pressure (hypertension) is also estimated today to cause some 7.5 million deaths annually accounting to about 12.8% of the total of all deaths. The changing lifestyle of our human race has further increased the prevalence of many other metabolic and age-related diseases which are too complex for therapeutic intervention via the one drug→one target→one disease approach. The 21st century complex diseases thus necessitate smart drugs that work through mechanisms far beyond the traditional modern therapeutic principle.
The merit of one smart drug targeting the various components of one or many complex diseases has become significance in recent years. One can imagine the befit of a multifunctional compound that lowers raised blood glucose level in the blood (hence antihyperglycemic) and at the same time target the associated inflammation, obesity or many other risk factors of diabetes. Such drugs acting through one drug→multitargets→one/many disease(s) principle could fit for purpose in tackling our 21st century complex diseases. Interestingly, perhaps some half a century ago, such pharmacological agents could have been considered as dirty drugs for them not being specific to one mechanism/target. The boundary line between smart and dirty drug is thus becoming less distinct when one considers treating complex diseases. Plant polyphenols belong to this category of secondary metabolites that offer general health benefits through non-specific antioxidant mechanisms and some specific actions against various cellular enzymes and other targets. Accordingly, establishing their multifunctional role to tackle diseases like diabetes/obesity along with many other complex diseases (e.g., Alzheimer’s disease) have been the focus of our research in recent years. Many review articles substantiating such drug-target approaches for complex diseases have also been published.11-21 Interestingly, many plant polyphenols are not only working through multifunctional mechanisms but also synergise to produce more pronounced effects. Hence, a crude drug preparation may offer a far better effect than isolated pure compound. We have demonstrated this principle (Multidrugs→multitargets→one/many disease(s)) in various purified and crude drug preparations and the concept should be considered as a viable option in treating diabetes/obesity or other complex diseases. For example, many flavonoids have been shown to offer antidiabetic potential through synergistic effect in a-glucosidase, a key carbohydrate digesting enzyme, inhibition in the gut.22 On the other hand, plant extracts which showed interesting pharmacological activities may sometime fail to give one or few active principles that account for the observed pharmacological activity suggesting the synergistic/additive effects of may compounds in the crude extract. A good example is that we have shown for the crude extract of corn silk (Zea mays) that offers a good anti-inflammatory activity in the form of a crude extract.23 Under this circumstance, the use of crude plant extracts instead of a single chemical entity should be advocated.
Our enthusiasm for discovering complex compounds that may offer novel structural moieties for new/better pharmacological effects has always been the major driving force to do more research in medicinal chemistry and natural products researches. There are numerous examples however where a drug molecule does not necessarily have to be big or complex to produce the desired effects. Aspirin is the classical example as with the numerous plant-derived phenolic compounds based on one aromatic ring system. Hence, gallic acid bearing optimised catecholic functional groups (Figure 2) is one of by far the most potent antioxidant compounds we have characterised to date in various biological systems both in vitro and in vivo. Increasing the structural complexity of these compounds does not also seem to offer more potency in many biological systems as we have demonstrated in several studies .24-40. Furthermore, gallic acid and its derivatives display numerous other pharmacological effects that make them potential drug candidates from anticancer/chemopreventive agents to complex metabolic disease inhibitors. Such structural features in one aromatic ring system; two/three rings of the flavonoids class and many other polyphenol forms thus make the compounds viable drugs in tackling complex diseases through various mechanism or therapeutic principles. Furthermore, these compounds that we often take for granted are also principal components of many plant medicines. It is therefore easy to envisage that, while simple multifunctional bioactive compounds of gallates and flavonoids classes could be sources of big disappointment to drug discovery scientists isolating them, they have tremendous potential as drugs in their pure form or as active principles of crude plant mixtures.
Overall, science allows modernising traditional plant medicines in many ways. The concept of a single chemical entity as a drug should not be seen as in conflict with herbal medicinal uses. Where possible, plants should be investigated to give active principles for utilisation as single chemical entities. The use of herbal medicines in their crudest form could also be modernised by using the identified active principles as quality control measures. The concept of addressing multiple targets with one or few combinations of active compound(s) is gaining more acceptances for chronic and complex diseases of the 21st century. Herbal medicines working through multifunctional mechanism or polypharmacology could therefore be repurposed for the growing demand through scientifically validated efficacy studies.
No funding of either internal or external sources have been used for this contribution.
Dr. Solomon Habtemariam is a Director of Herbal Analysis Services UK and Pharmacognosy Research Laboratories at the University of Greenwich, Chatham-Maritime, UK. Dr Habtemariam received his BSc degree in Biology (minor - Chemistry) from the University of Addis Ababa and his Master's degree (combined-studies) in Pharmacology and Phytochemistry from the University of Strathclyde, Glasgow, UK. He stayed on at Strathclyde to study at doctoral level, studying on drug discovery researches and obtained his PhD in this area of research. After a number of years in teaching and post-doctoral research at the Strathclyde Institute for Drug Research and Strathclyde University, he joined the School of Science, University of Greenwich in September 1998. Dr Habtemariam has been a leader of taught programmes and researches on bioassays & natural products-based drug development. The various researches that he has undertaken include the identification of novel compounds of natural origin with potential, antimicrobial, anticancer, anti-inflammatory, anti-diabetic and anti-obesity among others. He has published more than 150 scientific publications.
None.
The author declares that there are no conflicts of interest.

©2017 Habtemariam. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.