International Journal of
eISSN: 2381-1803


Mini Review Volume 6 Issue 5
1Lawson Health Research Institute, Canada
10Oregon Health & Science University, USA
11Department Psychiatry, Medical Director and founding Director of HG&H Pharmaceuticals (Pty) Ltd, South Africa
2Department of Psychiatry, University of Puerto Rico, USA
3Department of Psychiatry, University British Columbia: University of Victoria medical campus, Canada
4Department of Psychiatry, University of Western Ontario, Canada
5Nursing Program, University of Toronto Faculty of Nursing, Canada
6Biomedical Sciences Honors Program, University Western Ontario, Canada
7Kinesiology Honors Program, University Western Ontario, Canada
8Northern Ontario Medical School, Thunderba, Canada
9Formerly Director of Medical and Scientific Affairs, USA
Correspondence: Simon Chiu, Lawson Health Research Institute 268 Grosvenor Street; Lab FB125, London Ontario N6A 4V2 Canada
Received: March 12, 2017 | Published: May 1, 2017
Citation: Chiu S, Raheb H, Terpstra K, Vaughan J, Carrie A, et al. (2017) Exploring Standardized Zembrin® Extracts from the South African plant Sceletium tortuosum in Dual Targeting Phosphodiesterase-4 (PDE-4) and Serotonin Reuptake Inhibition as potential treatment in Schizophrenia. Int J Complement Alt Med 6(5): 00203. DOI: 10.15406/ijcam.2017.06.00203
Converging evidence suggests that Disrupted in Schizophrenia (DISC-1) is a recognized risk gene for schizophrenia. The interaction of DISC- with PDE-4 (Phosphodiesterase Subtype-4) plays a crucial role in neurodevelopment and neuro-inflammation in schizophrenia. Serotonin has drawn increased attention for its role in modulating cognition function. Hence dual targeting Selective Serotonin Reuptake site (SSRI) and PDE-4 may offer novel therapeutic paradigm towards augmenting antipsychotics in schizophrenia. Translational studies show that the family of mesembrane-related alkaloids isolated from the South African plant: Sceletium tortuosum have been shown to hit dual targets: SSRI and PDE-4 allosteric site. The promising findings warrant a proof-of-concept randomized controlled study Zembrin extract in schizophrenia.
Keywords:schizophrenia, disc-1 gene, serotonin, phosphodiesterase, zembrin extract
In schizophrenia treatment (SCZ) while the second generation of atypical antipsychotics are associated with fewer extra pyramidal motor side effects, the atypical antipsychotics prove to be no superior than the 1st generation of antipsychotics. The overall long term outcomes in area of social and vocational domains remain unfavourable.1 Cognitive impairment precedes the emergence of positive and negative symptoms and appears to persist throughout the course of the disorder.2,3 Expert consensus concludes that the negative outcomes are strongly related to the severity of impaired neuro-cognition (INC). The recent NIMH initiative, MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) outlines a strategic plan to address the issue through identifying promising molecular targets for future drug development.4,5 Pharmacological interventions targeting various neurotransmitter networks: GABA, glutamate, dopamine (DA), serotonin (5-HT), norepinephrine have met with mixed findings.6
The issue of enduring negative symptoms carries equal weight in determining the overall quality of life in schizophrenia population. The primary deficit syndrome, defined as a disease entity characterized by the presence of primary enduring negative symptoms, appears to be relatively refractory to the newer atypical antipsychotics.7 Primary negative symptoms are common in around 26% of the schizophrenia patient population and as high as 58% of the schizophrenia outpatients are estimated to experience the negative consequences of negative symptoms.8 A recent systematic review of the pharmacological treatments in negative symptoms.9 highlight the new approaches in targeting: 1) enhancing N-methyl-D-aspartic acid (NMDA) function through glycine binding site or glycine uptake inhibitor; 2) metabotropic glutamate receptor: mGluR2/3) allosteric modulator; 3) nicotinic cholinergic receptor. It has become a high priority to adopt a new approach to address the issues of neuro-cognition and negative symptoms. Recently, there has been increased interest in the emerging role of cAMP signaling in modulating neuro-cognition schizophrenia.10
DISC-1 gene and PDE: role in schizophrenia
Converging gene evidence suggests that the gene, Disrupted-in-Schziophrenia-1 (DISC1, MIM605210) is highly implicated as one of the important group of risk genes for schizophrenia.10,11 The DISC locus was originally discovered in a large Scottish family pedigree with a balanced translocation (q42.1, q14.3); the chromosomal translocation co-segregates significantly not only in SCH, bipolar disorder (BPD) and severe recurrent depression.12 Since then, various genetic association studies have replicated the original translocation family study. Genetic variants: single nucleotide polymorphisms (SNP) have been identified in a large sample from European population.13 A meta-analysis of 9 different schizophrenia samples from different European populations identified 50 SNPs and found evidence for a common interval with the DISC1intron 4-6 defining the schizophrenia risk.
Significant advances have been made to delineate the role of DISC-1 in encoding a multifunctional scaffolding protein that specifically regulates 2nd messenger: cyclic adenosine monophosphate (cAMP) signaling via interactions with phosphodiesterase 4 (PDE4).10,14 There is a growing body of evidence in support of the regulatory role of DISC-1in neurodevelopment and neuro plasticity.15 Dysregulation of synaptic plasticity due to SCZ at risk gene may be the underlying pathophysiology of SCZ. DISC-1 regulates neurogenesis, neuronal migration, dendritic arborization, and integration of cortical and hippocampal neurons. The family of phosphodiesterase hydrolyzes intracellular cAMP, More importantly, PDE4B has been shown to bind with DISC1.12 Two DISC-1 strains of mutant mice have been demonstrated to exhibit reduced binding capacity and affinity to PDE-4B while exhibiting behavioral changes resembling SCZ and depression symptoms.16
There has been growing interest in the crucial role of cAMP-response element binding protein (CREB), coupled with the intracellular second messengers: cAMP and cGMP, in regulating multiple gene expressions.17-19 Both cAMP and cGMP signal cascades are involved in neuronal survival, apoptosis, and synaptic strength and plasticity and neural network connectivity in CNS. In this respect the superfamily of 11 phosphodiesterases (PDE) widely distributed in the mammalian brain function regulate and fine-tune the homeostasis of cAMP-cGMP via sensing the intracellular levels of cyclic nucleotides.20 There are 11 members of PDE- family: PDE-1, PDE-2, PDE-3, PDE -4, PDE-5, PDE-6, PDE-7, PDE-8, PDE -9, PDE-10 and PDE-11 with their characteristic substrate-specificities, tissue distribution and physiological functions. The PDE family has a total of 21 isoforms with multiple gene splice variants that encode more than 50 proteins expressed in mammalian cells. There is in vitro evidence that release of DISC-1-mediated transcription repression of PDE4D9 serves as a paradigm of feedback inhibition to inhibit dopaminergic signaling.21 High levels of cAMP during stress exposure impaired prefrontal cortex (PFC) function and is consistent with the link of DISC1 with the negative impact of stress in neurodevelopment.22
PDE inhibitors are active in models of positive and negative symptoms of SCZ. PDE-4B knockout mice exhibited behavioral changes equivalent to negative symptoms and cognition deficits: decreased locomotor activity and attenuated prepulse inhibition accompanied by reduced dopamine and serotonin turnover.23 Transgenic mice with forebrain restricted expression of mutant human DISC1 (ΔhDISC1) showed abnormalities in oligodendrocyte (OLG) differentiation and OLG gene expression deficits.24 premature OLG differentiation and increased proliferation of their progenitors were identified. This finding suggests that PDE may be involved in remodelling of myelination defects in SCH.
Rolipram, the PDE4 inhibitor, improved memory consolidation, working memory and information processing in rodent species subject to a variety of cognitive tasks: radial arm maze, passive avoidance, delayed arm water and visual -spatial tasks, working memory and information.25-28 In the macaque monkey species, rolipram improved object retrieval performance suggesting positive interaction with executive function.26 Furthermore, rolipram antagonized phencyclidine (PCP)-induced hyperactivity and cognitive deficits, reversed amphetamine -induced pre- pulse inhibition and facilitated extinction of cocaine-induced place preference.29,30 The mechanism of the pro-cognitive action of PDE-4 inhibitor, rolipram, may be related to the co -expression of PDE-4 with DARPP -32 in D1 receptor-positive cortical pyramidal neurons in layer VI.31 Rolipram was active in the sensori-motor gating test.31 which may be explained by the enhanced DARPP-32 phosphorylation invoked by D1 receptor activation in the frontal cortex. Recent translational studies of PDE have also focused attention on PDE-10 and PDE-2A as putative targets for pro-cognitive strategy in SCZ and neurodegenerative disorders: Alzheimer dementia.33,34 None of the PDE -10A or PDE-2 is in Phase II/III clinical trials in SCZ. Taken together, elevated cAMP contributes towards cognition deficits mediated through the prefrontal cortex and targeting PDE-4- phosphorylated cAMP Response-Element-Binding protein (CREB) (19) can be beneficial in SCZ.
Converging evidence suggests that cognitive impairment in schizophrenia is related to neuro-inflammation. The anti-inflammatory action of the prototypal PDE-4 inhibitor, roflumilast, currently indicated for the treatment of chronic obstructive pulmonary disease (COPD) is relevant to our understanding of the emerging role of PDE-4 in cognition.35 Genetic knock- down of PDE-4D enzyme: PDE4d (-/-) mice displayed behavioral phenotype resembling anti-depressant treatment exhibited resistance to depression in the classical Porsolt force swim test and in the hyperactivity of the acoustic startle response.36 Rolipram exhibits anti-depressant effect and reverses Aβ-induced cognitive impairment and neuro-inflammatory and apoptotic responses in rats via PDE-4D or PDE-4B inhibition.37 Cyclic AMP signaling stands at the cross-roads of immune cell (microglia) activation and inflammation. In the glia, pro-inflammatory cytokines are involved in regulating PDE-4-cAMP signaling in the glia; whereas PDE-cAMP-cascade can in turn modulate cytokine production: the family of interleukins and alpha-tumor necrosis factor.38 The cross-talks between cytokine production and immune activation are part of the feedback loop coordinated through PDE-4-cAMP pathway.
Despite translational studies in support of the anti- psychotic efficacy of rolipram in schizophrenia and depressive disorders;, serious adverse effects of nausea and vomiting have largely limited further therapeutic development. A single dose-finding study in 6 schizophrenic patients found an initial improvement of psychotic symptoms during the first week; however, psychotic symptoms recurred in parallel with serious nausea and vomiting.39 Rolipram appeared to exhibit anti-depressant activity in major depressive disorder.40
Zembrin extract and PDE- 4 allosteric modulator/5-HT Reuptake inhibitor: In South Africa, extracts from South African succulent plant, Sceletium tortuosum, has been used for centuries as an orally available indigenous medicine, and for promoting well-being, enhancing mood and reducing stress.41-43 The standardized extract S, tortuous: Zembrin extract was developed by HG&H Pharmaceuticals Ltd. Bryanston, South Africa.
The cognitive enhancing effects of the key ingredients of Zembrin® extract are explained largely by the efficacy of alkaloids in competing for the PDE-4 binding. The chemical structures of mesembrenone alkaloids are fully characterized (Figure I). In the PDE assay system using the human recombinant PDE-4B expressed in Sf9 cells), the extract Selenium tortuous (Zembrin®) selectively inhibited phosphodiesterase 4 (PDE4) with IC50 (inhibitory constant) value of 8.5μg/ml. Mesembrenone, a major alkaloid isolated from the extract (Figure 1) was the most active in inhibiting PDE4 with an IC50 value of <1μM ( 44 ). Mesembrenone was 17 times more potent than mesembrine and 34 times more active than mesembenol. The IC50 values for mesembrenone were 470NM, followed by mesembrine: 7800nM and mesembrenol: 10,000 nM. Structure-Activity Relationship (SAR) studies show that cognition is likely to be selectively mediated via the long isoform of PDE-4D3.
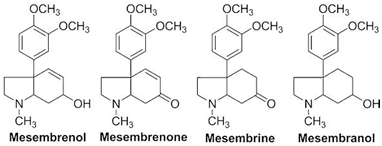
Figure 1 Chemical structures of alkaloids isolated from Sceletium tortuosum: mesembrine, mesembrenol, mesembrenone, mesembranol.
Mesembrenone alkaloids distinguish from other putative PDE -4 modulators in exhibiting concomitant serotonergic property. The serotonergic property of mesembrine alkaloids synergizes with cAMP signal pathway in mediating the cognitive effects. Mesembrine was found to be the most potent compound in binding to the serotonin transporter site (5-HT site) with Hill coefficient of 1.4 nM. Mesembrenone and mesembranol were found to be much weaker in the 5-HT transport site in vitro.44 The efficacy of Selective Serotonin Reuptake Inhibitors (SSRI) in the treatment of anxiety and depressive disorders is well documented.45 More recently escitalopram, the putative SSRI, has been shown to improve cognition and facilitate post- stroke recovery.46 Taken together, there is growing evidence to substantiate the role played by serotonin in decision making process within the cognitive domain of executive function.47 High central serotonin levels are functionally associated with improved reversal learning, enhanced attentional set shifting and response inhibition while reducing delay discounting. Preliminary evidence suggests that drug development platform based upon serotonin receptor subtypes: 5-HT (1), 5HT (4), and 5-HT (6) may hold promise for the treatment of schizophrenia.48
Rolipram, despite its efficacy in SCH and depression models, fail to advance to final phases of clinical trial because of the serious side effects of nausea and vomiting. Investigators explore matching the preferred conformation at the regulatory site of PDE-4 with the potential candidates in designing a series of PDE-4 allosteric modulators.49 It is perhaps fortuitous the HG & H Pharmaceuticals (Pty) Ltd discovered that the South Africa legendary plant, Sceletium tortuosum exhibited intrinsic activity as a PDE-4 allosteric modulator but devoid of serious emetic effects.41 In this respect, the family of mesembrane- related alkaloids from Zembrin extract find the respected niche in comparison with the group of patented PDE-4 allosteric modulators with therapeutic potential for the treatment of schizophrenia, Alzheimer’s dementia and depression: EHT0202 (etiolate hydrochloride).50 L-454,560.51 GEBR-7-b, derivative of rolipram.52
The functional MRI (fMRI) study conducted in Europe demonstrated for the first time that Zembrin passes through the BBB (Blood-Brain Barrier) to modify the functional responses towards environmental cues capable of generating fear.53 In a double-blind, placebo-controlled, cross-over design, 16 healthy participants were scanned during performance in a perceptual-load and an emotion-matching task. The reactivity of the brain region, amygdala, to fearful faces under low perceptual load conditions was attenuated after a single 25mg oral dose of Zembrin. Follow-up connectivity analysis on the emotion matching task showed that the amygdala-hypothalamus coupling was adversely affected. This fMRI study clearly shows that S. tortuosum (later formulated as the standardize Zembrin extract) attenuates the emotional responses towards subcortical threat circuitry. The result is consistent with the action of Zembrin extract in dual targeting SSRI and PDE-4 in the acute anxiolytic effects. Earlier, in the rodent model of restraint-induced anxiety and behavioral stress, S. Tortuosum at the lower dosage of 5-10 mg/kg when given by the oral lavage, exerted a marginal anxiolytic effect.54 Species differences in dose-response relationship of Zembrin may explain the discrepant findings.
Gericke.55 reported for the first time on three case studies of Zembrin in patients diagnosed as major depression disorder (n=2) and dysthymia with co-morbid personality disorder (n=1) and major depressive disorder. In the 1st case, the male patient responded to daily oral dosage of 50mg Zembrin tablet with significant sustained reduction in relatively severe depressive symptoms: insomnia, anhedonia, and suicidal ideation and mood fluctuations. During the first week, transient anxiety symptoms were found. In the second case, the female patient reported that “her mood had lifted, feeling more focused and less anxious” within 10 days of starting on 50mg Zembrin tablet. Her hypersomnia was also resolved, in the third case, the patient who was non-responsive to St. John’s Wort (hypericum perforatum) extract reported improvement in her mood. She noted that her hypersomnia amounting to 14 hr per day was changed to her normal pattern of 8hrs/day.
Recently, Chiu et al examined the cognitive effects of the standardized extract Sceletium tortuosum (abbreviated Zembrin@) in a RCT study of a cohort of cognitively healthy subjects ( n=21, mean age: 54.6 yrs) administered Zembrin extract, at the daily oral dosage of 25mg, for three weeks.56 The standardized extract Sceletium tortuosum (Zembrin®) was manufactured according to European Union Good Manufacturing practice (GMP).The extract was in the form of a fine dry powder with the dry plant material: extract ratio of 2:1, standardized to a total alkaloid content for the four main Selenium alkaloids (mesembrenone, mesembrenol, mesembrine and mesembranol) of 0.4%. The contents of the four alkaloids were quantified using High Pressure Liquid Chromatography (HPLC) analysis against validated analytical reference standards We demonstrated that Zembrin® treatment compared with the placebo group, selectively and significantly enhanced the cognitive measures of executive function (p< 0.032) and cognitive flexibility (p<0.022) in the computerized neurocognitive battery Vital Signs. Executive function encompasses higher-order cognitive processes: working memory, attention control, response inhibition and concept formulation, and is thought to be primarily driven by the prefrontal cortex. Zembrin® also improved processing speed, psychomotor speed and complex attention.
The subjective ratings of generalized well -being and positive mood states in our study.56 agrees well with a recent safety and tolerability study on Zembrin® in healthy subjects who reported “uplifted spirits” and better coping with stress and depressing events.57 Furthermore, subjects taking Zembrin® reported improvement in the subjective quality of their sleep on the HAM -D subscale: the mean rating of sleep onset at baseline, 0.76 (SD = 1.14), changed to 0.43 (SD =0.93) at week 3 (paired t-test, t = 2.09, df = 20, p=0.049, 2-tailed). In both studies.53,56,57 Zembrin® treated group found an overall improvement in the quality of sleep. In the three clinical studies in normal subjects.53 Zembrin® was safe and well tolerated. There were no changes in blood pressure, pulse, temperature and weight in either the Zembrin® group or the placebo group. Intriguing enough, the study led by Neil et al.57 found that the placebo group complained of increased frequency of adverse events than the Zembrin® group. The incidence of treatment emergent adverse events (TEAE) classed as “mild” and “moderate” was quite low
The pharmacological effects of Zembrin® in humans are consistent with the results of an EEG study in conscious freely moving rats.58 The paradigm of electropharmacogram (EPG) was used to characterize the actions of Zembrin®. As a novel neurophysiological technique, EPG consists of frequency analysis of the field potentials in the presence of the active drug. The electrical signals were transmitted via the wireless mode and processed using a Fast Fourier Transformation (FFT). The frequency ranges are sensitive to the specific neurotransmitter system and spectral power was evaluated over the 8 frequency ranges: delta, theta, alpha, beta1a, beta1b, beta2, gamma power. Zembrin® at the oral dosage of 2.5mg/kg, 5mg/kg and 10 mg/kg, significantly reduced alpha2 and beta1a which correlated with activation of dopaminergic and glutamatergic systems. Furthermore, Zembrin® attenuated the delta and theta frequency ranges which reflected changes in the cholinergic and norepinephrine systems as expected for drugs in neurodegenerative disorders. Zembrin induced the EEG changes typical of analgesic effects of standardized drugs being tested: theta wave reduction in common with the delta, alpha2 and beta1 attenuation. Zembrin® at 10mg/kg markedly attenuated theta waves which reflected the EEG effects of typical antidepressants.
In summary, the findings from the translational studies support the potential pivotal roles of PDE-4/cAMP/CREB cascade and serotonin in schizophrenia and provide the pharmacological basis of exploring Zembrin extract via dual targeting both PDE- 4 and serotonin in improving the negative symptoms and cognition deficits in schizophrenia. The paradigm of exploring Zembrin extract in targeting PDe-4/cAMP/CREB signaling may present a novel direction in regulating gene expression in schizophrenia (Figure 2). The synergy of serotonin and PDE-4 have spinoffs on the likelihood of resetting the D-1 activation and NMDA hypo function, and warrant the design of randomized controlled trial of Zembrin extract in the cohort of treatment refractory schizophrenia exhibiting persistent negative symptoms and cognitive deficits.
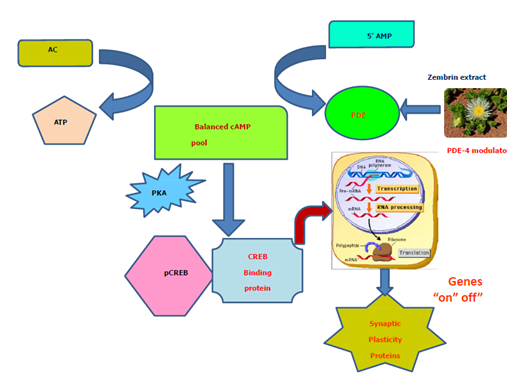
Figure 2 Model of PDE-4/cAMP/CREB Cascade in cognition.
Bi-directional regulation of cyclicAMP (cAMP) homeostasis is achieved through adenylate cyclase (AD) and phosphodiesterase (PDE) subtype 4. cAMP is activated via either PDE-4 inhibition or hormone/neurotransmitter-stimulated adenyclase cyclase (AD). The cAMP-dependent protein kinase A (PKA), once activated through allosteric site, can phosphorylate cAMP response element binding protein (CREB) to form phosphorylated CREB: pCREB. pCREB associates with transcription co-activator; CREB Binding protein to initiate transcription and translation. The CREB-mediated gene expression contributes towards long- and short-term memory and synaptic plasticity. Zembrin extract modulates PDE-4 and participates in PDe- 4/cAMP/CREB cascade in cognition. The CREB-linked gene expression has been shown to be impaired in AD model. Abbreviations: ATP (Adenosine triphosphate); cAMP (Cyclic Adenosine monophosphate), Adenylate cyclase (AC), 5’AMP (5’ adenosine monophosphate), CREB (cAMP Response Element Binding Protein); pCREB (phosphorylated CERB); PKA (Phosphokinase A)
None.
None.
The author declares that there are no conflicts of interest.

©2017 Chiu, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.