International Journal of
eISSN: 2381-1803


Research Article Volume 15 Issue 2
Department of Biology, School of Natural Sciences, University of Patras, Greece
Correspondence: Katsoris Panagiotis, Department of Biology, School of Natural Sciences, University of Patras, Greece
Received: March 11, 2022 | Published: March 28, 2022
Citation: Sarametidis P, Kourelis T, Bokea K, et al. Evaluating “Helleborus cyclophyllus” extract’s cytotoxic activity against malignant and normal cell lines· preliminary evidence of gastric acid resistance, and a promising therapeutic window. Int J Complement Alt Med. 2022;15(2):116-120. DOI: 10.15406/ijcam.2022.15.00599
Helleborus cyclophyllus is an ancient pharmaceutical plant that exhibits remarkable in vitro cytotoxic activity against malignant cell lines. In the present study, we evaluated the cytotoxic activity of Helleborus cyclophyllus dried powdered rhizome’s aqueous extract in both malignant (PC3, TPC1, K1 ) and normal ( Nthy-ori 3.1) cell lines. Our results demonstrated a promising therapeutic window, as normal cell lines exhibited significantly higher (x 10) IC50 than malignant cell lines. Gastric acid did not adversely affect Helleborus cyclophyllus extract’s cytotoxic activity as previous incubation in 1.0 M HCl acid did not result in IC50 values escalation. Finally, high extracellular lactic acid concentration resulted in Helleborus cyclophyllus extract’s cytotoxic activity amplification, whereas the presence of sodium lactate adversely affected cytotoxicity, revealing a possible indirect potential role for aerobic glycolysis to the clinically observed inadequacy of per os Helleborus cyclophyllus administration in cancer treatment.
Keywords: helleborus cyclophyllus, cytotoxicity, therapeutic window
Natural products and related drugs are used to treat more than 85% of all categorized human diseases including bacterial infection, cancer, and immunological disorders.1 About 25% of prescribed drugs in the world originate from plants and over 3000 species of plants have been reported to have anti-cancer properties.2,3 Natural products have been used since ancient times for the treatment of many diseases. Before the 20th century, 80% of all medicines used to treat human and animal illness were obtained from the leaves, barks, and roots of medicinal plants.2 It is noteworthy to mention that about 70% of the drugs used today are models of natural products.4 In the last 40years, most of the newly discovered chemical entities from natural products were anticancer drugs being isolated from either flora plants or fauna.3
Helleborus cyclophyllus, formally classified as [Helleborus Odorus Waldst & Kit subspecies Cyclophyllus (A. Brawn) Maire &Petitm] (Figure 1), is a Greek flora’s endemic pharmaceutical plant.5
Methods of processing the plant and the dried powdered root’s maximum tolerated per so dose in humans have well been established since antiquity.5 However, it has become obsolete in nowadays evidence-based medicine due to its unacceptable high risk-benefit ratio regarding its main therapeutic indications i.e. constipation and agitation.5 Nevertheless, its rhizome extract exhibits very promising cytotoxic activity against cancer cell lines.6–9 Therefore, determining the chemotype of its dried powdered root has become an increasingly important task of interest in the area of analytical chemistry.6,10,11 It seems that mainly responsible for the rhizome’s cytotoxic activity is the cardiotonic steroid hellebrigenin and its glycosidic analog hellebrin.11,12 Cardiotonic steroids exhibit an extremely attractive in vitro cytotoxic activity against both malignant and normal cell lines demonstrating a very promising therapeutic window as they selectively induce apoptosis rather to cancer than to normal cells.13–17 Numerous naturally isolated and semi-synthetic derivatives of cardiotonic steroids and hellebrigenin/hellebrin have been evaluated regarding their anti-proliferative activity during the process of drug development for cancer treatment.18 Retrospective statistical data have supported the cancer-preventing action of cardiotonic steroids.19 Unfortunately, subsequent clinical trials failed to demonstrate any clinical benefit.20 Nevertheless, informally in terms of alternative medicine and outside of the setting of a clinical trial, among metastatic cancer Greek patients, exists a prevalent common belief or superstition, that daily per os administration of Helleborus hyclophyllus dried powdered root may result in disease remission. However, there has never been reported, any evidence-based documented clinical regression, among health professionals in Greece.
In the present study, we evaluated the cytotoxic activity of Helleborus cyclophyllus rhizome extract against both malignant and normal cell lines. Furthermore, as preceding un succeeded clinical trials involved almost exclusively per os administration of cardiotonic steroids, in an attempt to demonstrate potentially counteracting factors regarding the per os route of administration, we tested if gastric acidity adversely affects dried powdered root’s cytotoxic activity. Finally, regarding the aerobic glycolysis over activated malignant tumor, lactic acid- abundant, microenvironment we evaluated whether high extracellular lactic acid or sodium lactate concentration inhibits dried powdered root’s cytotoxic activity against cancer cell lines.
H. odorus subsp. cyclophyllus A. Brawn Maire and Petitm, mother plants were harvested from the Greek flora (N 39°22.199' E 021° 14.109'-110' Altitude: 861–862m) and were subsequently submitted to clonization at the Holly and Great Monastery of Vatopaidi in Mount Athos, Greece. As a result, certified H. odorus sub.sp. cyclophyllus pharmaceutical plant clones were developed. The H. odorus sub.sp. Cyclophyllus ‘Vatopaidi’ (Italia Herbarium depository number: 2017.012 Helleborus οdorus Waldst. & Kit. subsp. cyclophyllus A. Brawn Maire and Petitm) was used as the experimental material.
Helleborus water extract. Air-dried roots from Helleborus cyclophyllus were grounded using an electrical grinder, and 400mg of powder was mixed with distilled H2O to a final volume of 10ml. The powder suspension was incubated for 60min at 40C on a rocker plate and centrifuged at 2,500g for 5min. The supernatant was filtered through Corning syringe filter (0,2μm pores) and stored at 40C until use.
Cell lines
Human Prostate Adenocarcinoma PC3 cell line was supplied from ATCC. Human Thyroid Papillary Adenocarcinoma TPC1, Human Papillary Thyroid Adenocarcinoma K1, and Normal Human Primary Thyroid Follicular Epithelial Cells Nthy-ori 3-1 cell lines, were supplied from Sigma-Aldrich.
Cell culture
All cells were grown PC3 Cells Nthy-ori 3-1 in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 100U/ml penicillin, and 100μg/ml streptomycin. Cultures were maintained in 5% CO2 and 100% humidity at 370C. K1 DMEM.
Crystal violet assay
Adherent cells were fixed with methanol and stained with 0.5% crystal violet in 20% methanol for 20min. After gentle rinsing with water, the retained dye was extracted with 30% acetic acid, and the absorbance was measured at 595nm. The cell number was calculated using a standard curve. The concentrations of the tested samples required to inhibit 50% cell proliferation (Half-maximal inhibitory concentrations, IC50), were calculated from the mean values of data from wells.
Statistical analysis
Comparison of mean values among groups was done using ANOVA and the unpaired Student t-test. Homogeneity of variance was tested by Levene's test. Each experiment included at least triplicate measurements for each condition tested. All results are expressed as the mean ± SD of at least three independent experiments. Values of p less than 0.05 were taken to be significant (***p < 0.001).
Cytotoxic action of HWE
To test the cytotoxic action of HWE, we used two cancer (PC3 and K1) and one normal (Nthy-ori 3.1) cell line, and incubated the same number of cells with increasing concentrations of HWE. As can be seen in Figure 2, HWE shows a cytotoxic activity in all cells, but with different IC50. Comparing the normal Nthy-ori 3.1, with cancer cell line K1 (both are thyroid cells) is clear that the normal cells are more resistant than the cancer ones. Each experiment included at least triplicate measurements for each condition tested. All results are expressed as the mean ± SD of at least three independent experiments.
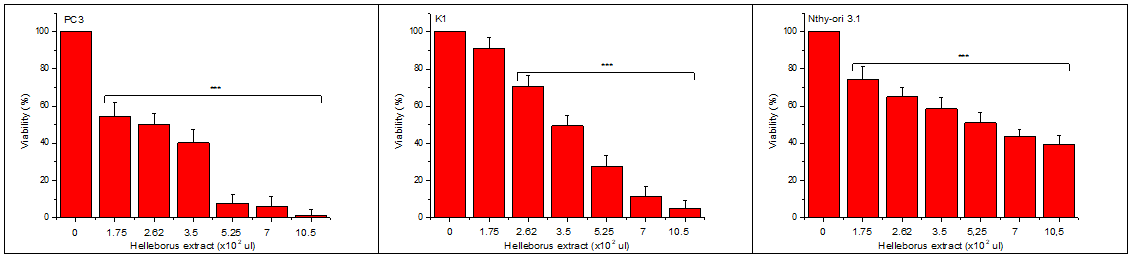
Figure 2 Cytotoxic action of HWE (Helleborus water extract).
An equal number of cells were transferred to microplate wells and incubated with increasing final concentrations of Helleborus extract in a complete medium. After 48 hours, the number of viable cells was estimated using the crystal violet assay (see Materials and Methods).
Lactic acid increases the cytotoxic activity of HWE
Since the solid tumor environment is rich in lactic acid, we evaluated the action of HWE in presence of lactic acid. We incubated the same number of cells with two concentrations of HWE in the presence or not of lactic acid in two different concentrations. As can be seen in Figure 3, lactic acid per se has no cytotoxic activity, but in contrast, it increases in a dose-dependent manner the cytotoxic activity of HWE. Each experiment included at least triplicate measurements for each condition tested. All results are expressed as the mean ± SD of at least three independent experiments.
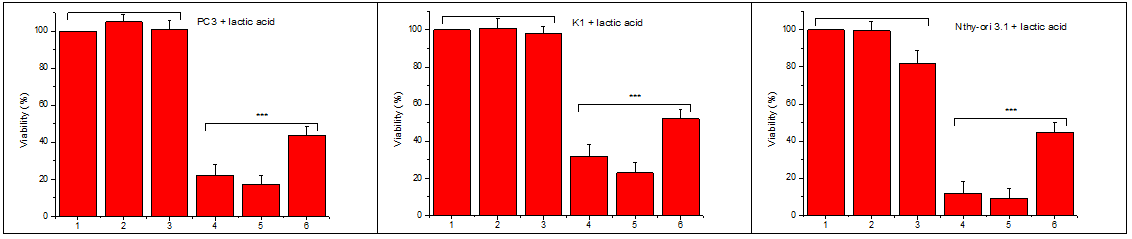
Figure 3 Lactic acid actions on the cytotoxicity of Helleborus water extract (HWE).
An equal number of cells were transferred to microplate wells and incubated with increasing final concentrations of lactic acid, or with increasing final concentrations of lactic acid and IC50 HWE in a complete medium. After 48hours, the number of viable cells was estimated using the crystal violet assay (see Materials and Methods).
1: Control, 2: 10mmol/l lactic acid, 3: 30mmol/l lactic acid, 4: IC50 HWE+ 10mmol/l lactic acid, 5: IC50 HWE+ 30mmol/l lactic acid, 6: IC50 HWE.
Sodium lactate does not effect on the cytotoxic activity of HWE
Similarly, with lactic acid, we tested the action of sodium lactate. We incubated the same number of cells with two concentrations of HWE in the presence or not of sodium lactate in two different concentrations. As can be seen in Figure 4, sodium lactate per se has not cytotoxic effect and does not differentiate the cytotoxic activity of HWE. Each experiment included at least triplicate measurements for each condition tested. All results are expressed as the mean ± SD of at least three independent experiments.
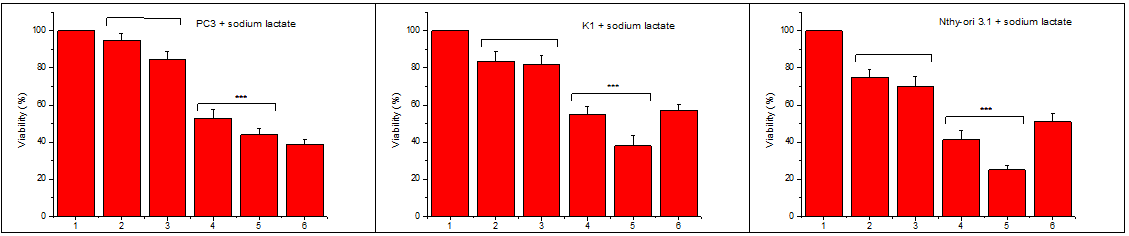
Figure 4 Sodium lactate action on the cytotoxicity of Helleborus water extract (HWE).
An equal number of cells were transferred to microplate wells and incubated with increasing final concentrations of sodium lactate, or with increasing final concentrations of sodium lactate and IC50 HWE in a complete medium. After 48 hours, the number of viable cells was estimated using the crystal violet assay (see Materials and Methods). 1: Control, 2: 10mmol/l sodium lactate, 3:30mmol/l sodium lactate, 4: IC50 HWE+ 10mmol/l sodium lactate, 5: IC50 HWE+ 30mmol/l sodium lactate, 6: IC50 HWE.
Our experiments proved the anti-cancer potential of the water extract of Helleborus’ root in thyroid (K1 and TPC1) and prostate (PC3) cancer lines, as well as in normal cells (Nthy-ori 3.1 cell line and Healthy donor’s leucocytes ). Moreover, our results demonstrate a promising therapeutic window, as normal cells proved to be at least two and a half times more resistant to the cytotoxic action of Helleborus cyclophyllus extract.
Gastric acidity did not affect the cytotoxic potential of Helleborus cyclophyllus extract as previous acidification with 120 min incubation in HCl acid 1M pH 1.0 and subsequent restoration had no impact on IC50 values.
Lactic acid proved to act synergistically, as high lactic acid concentrations in culture media intensified the cytotoxic potential of Helleborus Cyclophyllus extract.
Sodium lactate adversely affected Helleborus Cyclophyllus cytotoxic activity in PC3 cell line as we observed an inhibition of up to 15% ( at 10mM/L sodium lactate with the corresponding for these cells IC50). However, sodium lactate had no impact on K1 cell line IC50. Moreover, regarding Nthy-ori 3.1 cell line, we observed a small increase in mortality at each sodium lactate concentration.
Helleborus cyclophyllus rhizome’s extract exhibits differential cytotoxicity against malignant and normal cell lines, with normal cell lines IC50 being remarkably (at least x 2.5) higher than malignant cell lines IC50. Obviously, it is preliminary evidence of a therapeutic window as cancer cells are more susceptible to Helleborus Cyclophyllus induced cell death than normal cells. Moreover, the gastric environment proved to be harmless as both previous 60-min incubation in pH 1.0 HCl failed to increase IC50. Finally, high extracellular lactic acid concentration seemed to act synergistically by further reducing IC50 in both malignant and normal cell lines, whereas sodium lactate presence increases PC3 cell line IC50, but did not affect K1 cell line IC50. On the contrary, regarding Nthy-ori 3.1 cell line, high sodium lactate potentiated the cytotoxic activity of Helleborus Cyclophyllus extract, which might be considered reasonable as Nthy-ori 3.1 is a normal cell line. Therefore, regarding Helleborus Cyclophyllus cytotoxic activity, high extracellular sodium lactate concentration might be an inhibitory factor. Mainly responsible for the dried powdered root’s extract cytotoxic activity is the bufadienolidic cardioactive steroid Hellebrigenin and its di-glycoside analog Hellebrin.11,12 Hellebrigenin/Hellebrin pair is the sine qua non of the hellebores dried powdered root’s chemotype since it is invariably present in 0.5%-1.5 % w/w concentration range.5,6 Cardioactive steroids exhibit a very promising therapeutic window as they selectively induce apoptosis in cancer cells rather than normal cells, via binding to the cardioactive steroid-binding pocket on the extracellular segment of the transmembrane caveolae Sodium/Potassium ATPase pump.13–17 From that point of view, our results regarding dried powdered root’s extract therapeutic window are in accordance with cardioactive steroids - induced, malignant cell-selective documented, cytotoxicity.13–17 Unfortunately, clinical trials involving per os cardioactive steroids administration in cancer patients failed to demonstrate any clinical benefit.20 A possible explanation could have been that low gastric pH might irreversibly alter the molecular structure and adversely affect the cytotoxic activity of cardioactive steroids. However, our results indicate that gastric acidity is not responsible for, per os cardioactive steroids administered, clinically observed inadequacy regarding malignant tumor regression. From that point of view, our results are following the fact that the aromatic nature of delta-valerolactone bufadienolidic ring renders it not amenable to the corresponding levulinic acid even in extreme pH values. Another possible explanation regarding the clinically documented therapeutic inefficiency of cardioactive steroids in cancer treatment might rely on the lactic acid abundant tumor microenvironment due to aerobic glycolysis overactivation.21 At a first glance, one would speculate that aerobic glycolysis is not an impending procedure since high in vitro extracellular lactic acid concentration acts synergistically by further enhancing Helleborus Cyclophyllus- induced cytotoxicity. However, since sodium is the main in-vivo extracellular highly concentrated ion, another possible explanation could be that the intracellularly, aerobic glycolysis - overproduced, lactic acid may extracellularly be conjugated to sodium and therefore instantaneously resulting in sodium lactate formation. From that point of view, since lactic acid renders extracellular pH slightly acidic, whereas sodium lactate renders it slightly alkaline, our results, regarding the cytotoxic inhibitory activity of sodium lactate in contrast to the cytotoxic promoting activity of lactic acid, are following previous studies revealing an amplifying role of acidic pH and an inhibitory role of alkaline pH in the binding of cardioactive steroids to the Sodium Potassium ATPase cardioactive steroid extracellular binding pocket.22 Based on that speculation it would be possible that a future clinical trial involving combination therapy with coadministration of cardioactive steroids and aerobic glycolysis inhibitors might yield favorable clinical outcomes in cancer patients.
None.
The authors declare no conflicts of interest.
There was no funding received for this paper.

©2022 Sarametidis, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.