International Journal of
eISSN: 2381-1803


Research Article Volume 12 Issue 4
1Department of Pharmacy, Jashore University of Science and Technology, Bangladesh
2Executive, Product Development, Aristopharma Ltd, Bangladesh
3Department of Pharmacy, State University of Bangladesh, Bangladesh
4Department of Pharmacy, East West University, Bangladesh
Correspondence: Mushiur Rahman SM, Department of Pharmacy, Jashore University of Science and Technology, Jashore-7408, Bangladesh , Tel 8801632533567
Received: June 24, 2019 | Published: August 30, 2019
Citation: Rahman MSM, Rana S, Islam AAFM, et al. Antithrombotic and anti-inflammatory activities of leaf methanolic extract of Euphorbia hirta Lin. Int J Complement Alt Med. 2019;12(4):154-162. DOI: 10.15406/ijcam.2019.12.00466
Euphorbia hirta (Euphorbiaceae family) has been claimed as a useful folk medicine to traditional healers for the treatment of fever, pain, diarrhoea, peptic ulcers, vomiting, asthma, bronchitis, inflammation, kidney stones, and menstrual problems and as anti-venom. Therefore, the intention of our study was to evaluate the possible antithrombotic and anti-inflammatory activities of methanolic extract of Euphorbia hirta leaves in mice. The in-vitro clot lysis test was performed to investigate the antithrombotic activity of Euphorbia hirta leaf methanolic extract. In addition, xylene-induced ear edema and cotton pellet-induced granuloma formation tests were conducted to evaluate the anti-inflammatory effect of methanolic extract of Euphorbia hirta leaves in mice. Crude methanolic extract was administrated orally at a dose of 200, 400 and 800mg/kg in mice. Treatment with crude extract of Euphorbia hirta leaves (100 and 200mg/mL) restrained the in-vitro thrombosis process. In this test, MEHL 200mg/mL showed the strongest inhibition value (38.00±2.52 %) of thrombosis. The treatment with MEHL at a dose of 200, 400 and 800 mg/kg showed a dose-dependent significant inhibition of ear edema in mice, whereas the highest inhibition value was exhibited by MEHL 800mg/kg (58.78±2.90%). Moreover, all of the dosages (200, 400 and 800mg/kg) of MEHL were significantly reduced the granuloma formation in mice as well as decrease the body weight gain. Here, MEHL 800mg/kg provided highest inhibition value (56.80±3.85 %) of granuloma formation in mice. Furthermore, no sign of toxicity, behavioral changes or mortality was observed upto the dose of 3200 mg/kg for MEHL or control group. The present investigation explores that methanolic extract of Euphorbia hirta leaves has moderate antithrombotic and significant anti-inflammatory effects. These outcomes strongly support the traditional uses of this plant scientifically, in case of thrombosis and inflammation management.
Keywords: Euphorbia hirta, thrombolysis, anti-inflammatory, cotton pellet
Nature is the root-source of trustworthy remedies and gifted us plenty of beneficial herbs, vegetables, fruits and medicinal plants which are the reliable resource of traditional medicines used to cure illness widely, since the primitive period of time.1 Natural medicines based on medicinal plants are a renowned source of bioactive components which are willingly used for the discovery and development of unique therapeutic agents and the effective management of a broad range of ailments.2
Current scientific statistics substantiated that cardiovascular diseases along with thrombosis are the most couple leading causes of world population death.3 The formation or emergence of thrombus in an artery or vein which is attributed to platelet adhesion and aggregation, blood vessel injury, endogenous and exogenous coagulation system, and the yield of fibrin is acquainted as thrombosis.4 Generally, thrombosis is the most complicated multifactorial pathologic process which is triggering numerous diseases including deep vein thrombosis, ischemic myocardial infarction, atherosclerotic plaques, and sudden death. In addition, thrombosis strongly damages the quality of human life and responsible for much morbidity and mortality.5, 6 The intravascular thrombosis is one of the main reasons for cardiovascular disease and approximately 99% infractions are caused by embolic or thrombotic events.5 Pharmaceutically manufactured antithrombotic armamentarium such as aspirin, streptokinase, warfarin, and clopidogrel reveal some remarkable adverse effects and might explain the vascular relapses. Gastrointestinal bleeding, prolonged bleeding time, internal bleeding, headache, and palpitation are frequently occurring unexpected effects.7 For these reasons, it is highly required to exploration of novel bioactive compounds and medicines from medicinal plants with effective and precise mechanisms of action, therapeutically effective, and less toxicity.3
It has always been a conundrum for mankind to understanding inflammation literally. It plays a crucial role in the onset and progression of numerous conditions which is elicited by various noxious stimuli such as antigen-antibody interaction, infectious or aggressive agents (pathogens, irritants, or damaged cells), myocardial infarction and physical or thermal injury8,9 Inflammation is considered as an unpleasant complex biological condition which mediated by a number of signaling molecules generated by macrophages, leucocytes and mast cells.10 The release of arachidonic acid and inflammatory mediators such as prostaglandin, histamine, serotonin, cytokines, and leukotrienes increase the vascular permeability, as well as facilitating the migration of leukocytes to the site of inflammation.11,12 There is an inter-correlation between inflammation and pain resulting from the release of analgesic mediators.9 Commercially available anti-inflammatory drugs such as- steroidal, nonsteroidal (NSAID’s) and corticosteroids at high dosage or prolonged use can provoke some undesired and serious side or adverse reactions including osteoporosis, aggravation of ulcers, serious infections,13 peptic ulceration, epigastric distress and iatrogenic Cushing's syndrome.8 In addition, the rising costs of orthodox medicines are difficult to beard by most people of Bangladesh, especially in the rural parts where the greater percentage of the people are poor and merely subsisting. Consequently, development and establishment of non-toxic along with more powerful and cost-effective anti-inflammatory drugs that can promote a reduction in inflammation process is still needed.
Prominent qualitative compounds yield from medicinal plants such as alkaloids, flavonoids, saponins, glycosides, coumarins, and terpenoids could provide an excellent fountainhead to discover and develop new anti-inflammatory drugs.8
Euphorbia hirta L. (belongs to Euphorbiaceae family) is a resourceful plant which has a number of tremendous uses as medicine. Endemically this plat is known as dudhani, dudh ghas, baridudhi, dudeli, asthma plant, dudhi and considered as the biggest genus of this family.14,15 This is a small annual broad-leaved herbaceous wild plant native to Australia and commonly found in tropical countries,16 especially in Bangladesh and India. Usually, it is erect, slender-stemmed, annual hairy plant with number of branches from the base to top which spreading up to 40cm in height, though sometimes it can be seen lying down, reddish or purplish in color. Leaves are elliptical, opposite, oblong-lanceolate or simply oblong, acute or subacute, pale beneath, dark green above, 1- 2.5cm long, with a faintly toothed edge and blotched with darker or purple on the middle or upper surface.14,17 The flowers are generally small, about 1- 1.5cm in diameter, numerous and crowded together in dense cymes. The leaves and stems of E. hirta produce a milky-white juice when cut. It is frequently seen growing open waste spaces, banks of watercourses, pathways, roadsides, and grasslands.17,18
Notable chemical compounds of E. hirta are flavonoids (quercetin, myricitrin, rhamnose, rutin, quercitrin, quercitol, leucocyanidin, leucocyanidol, pelargonium 3,5-diglucoside, cyanidin 3,5-diglucoside), terpenoids (α-amyrin, β-amyrin, β-sitosterol, campesterol, friedelin, taraxerol, cholesterol, and stigmasterol), phenols, essential oil, tannic, ellagic, gallic, maleic and tartaric acid and other compounds.19,15
Traditionally, it is a very popular and trustworthy herb amongst practitioners in folk medicine and widely utilized as a palliative to treat various ailments including fever, intestinal parasites, diarrhoea, peptic ulcers, vomiting, amoebic dysentery, itching, asthma, emphysema, bronchitis, coughs, hay fever, laryngeal spasms, colds, wounds, kidney stones, menstrual problems, sterility abscesses and venereal diseases. In addition, the plant has a well-known reputation as an analgesic to treat severe headache, toothache, rheumatism, colic, and pains during pregnancy. To relieve the pain of scorpion stings and snakebites it is used as an effective antidote. This plant is also used by nursing mothers with deficient milk supply.19,20 Moreover, the anti-inflammatory, analgesic, antipyretic, sedative, anxiolytic, antifertility, anthelmintic, antiplasmodial, antiamoebic, antimalarial, larvicidal properties of E. hirta have been reported in the literature.16,17
Therefore, based on the need to establish more effective treatments with considerably fewer side-effects and more safety for thrombosis and inflammation to conventional pharmacotherapy and taking into account the therapeutic potential of the Euphorbia hirta. Moreover, herbs are more efficacious, safe, economic, and reliable, less toxic and accessible natural resource of drugs all over the world. So, the present study is designed to investigate the possible antithrombotic and anti-inflammatory activities of leaf methanolic extract of Euphorbia hirta plant.
Chemical reagents and drugs
Streptokinase and Diclofenac sodium were used as reference drug in this study and purchased from Beacon pharmaceutical Ltd., and Square Pharmaceuticals Ltd., Bangladesh, respectively. All chemicals and reagents used in these experiments were obtained from Merck, Germany of analytical grade and highly pure.
Plant collection and identification
With the aid of a comprehensive literature review, Euphorbia hirta (Euphorbiaceae) was selected for this investigation known as antithrombotic and anti-inflammatory activities. Freshness leaves of this plant were obtained from the green campus of Jashore University of Science & Technology (located at 23° 14' 0" N, 89° 7' 31" E), Jashore, Bangladesh, in September 2018. The plant was botanically identified and authenticated by the experts of the National Herbarium, Bangladesh and the voucher specimen number is DACB- 46543. A sample of dried specimen was archived in the herbarium for future reference.
Extract preparation
For the current investigation, approximately 300gm. of pulverized leaves were taken. First, the leaf part of the plant was collected and thoroughly washed 3-4 times successively with fresh current water and once with distilled water to remove all adhering substances (dirt, soil, and contaminants). The leaves were air dried at room temperature (25±2°C) for about two weeks and finally dried them for 24hours at 30±2°C by using a laboratory dryer (memmert UN75, Germany) prior to ground. Then all dried leaves were ground into coarse powder and cold extraction method was applied to extract the active components. Pulverized leaves (300gm.) were macerated with a sufficient amount of analytical graded methanol (1800mL) for 10-12days at room temperature with periodical shaking and stirring. Finally, the whole mixture was primarily filtered through fresh cotton and then through Whatman No.1 filters. After filtration, the greenish solvent was evaporated at reduced temperature (not more than 40°C) to yield concentrated crude extract. The percentage yield of the crude extract was 4.19% (w/w). The crude semisolid extract was then preserved in a refrigerator (at 4°C) till further studies.
Experimental animals
The test animals were procured from the Department of Pharmacy, Jahangirnagar University, Savar, Dhaka, Bangladesh. To run this study, 4-5weeks aged sixty male young Swiss albino mice of body weight 20-27gm. were used. Animals were kept under standardized environmental conditions, maintained 55%–65% relative humidity and exposed to alternative 12:12hours light and dark cycle at an ambient temperature of 26±2ºC before initiating these experiments. The animals were housed in plastic (polypropylene) cages with five mice in each cage, proper supplies of nutritional foods and water ad libitum was ensured. All protocols for the animal experiment were examined and endorsed by the Institutional Animal Ethical Committee of Jashore University of Science and Technology, Jashore, Bangladesh. Animals were habituated for 8-10days in our laboratory environment prior to experiment. Constant environmental and adequate nutritional conditions were ensured during the period of experiment.
Antithrombotic study
Collection of human blood: To conduct the in-vitro antithrombotic study, 2mL of human blood was collected from each healthy volunteer (n=3) without a history of oral anticoagulant therapy and all donor was completely acquainted about this experiment.
Procedure: The antithrombotic effect of MEHL was evaluated by the method established by Daginawala,21 and slightly modified by Kawsar 22. Briefly, 2mL of pre-collected venous blood was distributed into four different previously weighed sterile micro-centrifuge tubes (0.5mL/tube) and incubated at 37°C for 45min. Serum was completely removed after clot formation without disturbing the clotted blood. The colt containing each micro-centrifuge tube was reweighted to calculate the colt weight by the following formula.
Clot weight = (Weight of clot containing tube - Weight of empty tube).
Then, 100μL of methanolic extract of both dosages (50 and 100mg/mL) of Euphorbia hirta leaves was added in each tube separately. Here, commercially available streptokinase (lyophilized Altepase vial of 15,00,000 I.U) was used as the reference drug. Total 5ml of sterile distilled water was added with streptokinase vial and mixed properly. This suspension was used as a standard from which 100μL (30,000 I.U) was used for in vitro thrombolysis. 100μL of distilled water was used as a negative control and added to the negative control tube numbered. All microcentrifuge tubes were again incubated at 37°C for 90min. After completed the second incubation, released fluid was carefully removed from all tubes and further weighed to determine the difference in weight after clot breakdown.
Total of three replicates (n=3) of each sample was used for statistical analysis and values are represented as mean ± SEM (Standard Error of Mean). The percentage of clot lysis was calculated by the following standard formula.
Clot lysis (%) = (Weight of tube before clot lysis - Weight of tube after clot lysis) x 100
Acute oral toxicity study
To select the rational administrative dosages that are safe, non-toxic and most effective, the acute oral toxicity study was performed in compliance with OECD-423 guidelines, acute toxic class method.23 In total, ten healthy Swiss albino mice were divided into two groups, containing five mice in each group. MEHL was administered orally in the dose range of 3200, 1600, 800, 400, 200 and 100mg/kg body weight, randomly. The animals were kept fasting overnight providing only water ad libitum. Subsequent to administration of the extracts, the animals were observed closely for the first 3h for any toxic manifestations such as pain, noisy breathing, mortality, diarrhea, convulsion, salivation, weakness, changes in locomotor activity, aggressiveness, discharge from eyes and ears, injury, food or water refusal, coma and death. Subsequent observations were made at regular intervals for 24h. The animals were observed for a further week.24 The methanolic extracts were safe up to a dose of 3200mg/kg body weight, so 200mg/kg 400mg/kg and 800 mg/kg were used as moderate dose for the evaluation of anti-inflammatory activities.
Anti-inflammatory study
Xylene-induced-ear edema test: The acute anti-inflammatory activity of methanolic extract of Euphorbia hirta leaves was evaluated by xylene-induced ear edema in mice. An established method described previously by Dai&Liu25 was followed here with slightly modified form. Consisting of five mice in each group, twenty-five mice were randomly divided into five groups and had been fasted for 16h with water ad libitum. Group I was orally treated with distilled water (10mL/kg body weight) as negative control. Mice of group II were orally treated with Diclofenac sodium (DS, 100mg/kg body weight) and considered as positive control or standard. Test groups (group III and group IV) were orally administered with MEHL at dosages of 200, 400 and 800mg/kg body weight. Acute ear edema was induced by topical application of 20𝜇L of xylene on the anterior and posterior surfaces of the right ear lobe of each animal, after one hour from respective treatment. The left ear was untreated and regarded as control. Mice were sacrificed (1hour later of xylene application) by cutting off both ears with the utilization of 5 mm circular sections. Finally, the cut portion of each ear was seized and weighed accurately. The weight of ear edema was calculated from the difference between the weight of xylene treated ear (right ear) and the weight of untreated ear (left ear).26
The percentage inhibition of ear edema promoted by topical xylene application was determined by the following formula.
Inhibition (%) = 1- {Weight of edema (extract or standard drug)/Weight of edema (normal control)} X 100
Cotton pellet-induced granuloma formation: To search for a new anti-inflammatory agent, cotton pellet-induced granuloma formation in mice is used widely as a valid working model of inflammation. To conduct the cotton pellet-induced granuloma formation in mice, the method established by Swingle&Shideman,27 and with slightly modified by Rahman was performed.24 Mice were anaesthetized with light chloroform under aseptic condition. Sterile pre-weighted cotton pellets, weighing (10±1) mg of each pellet, were implanted subcutaneously, one on each side of the abdominal (axilla region) of the mice. Healthy twenty-five Swiss albino mice were divided and treated orally as mentioned previous experiment, once a day for seven consecutive days. After completed the routine treatment, all mice were anaesthetized again and dissected on the 8th day. Granuloma wetted cotton pellets were removed and dried for 24h at 60 with a laboratory dryer (memmert UN75, Germany). Finally, the dry cotton pellet weight was recorded. The net weight of granuloma formation was calculated by the weight difference between the dried cotton pellets and the cotton pellets before insertion.
The percentage inhibition of granuloma formation in mice generated by cotton pellet implantation was calculated by the following formula.
Inhibition (%) = 1- {Weight of granuloma (extract or standard drug)/ Weight of granuloma (normal control)} X 100
Statistical analysis
Experimental data are presented as mean ± SEM (standard error of mean). For the statistical assessment, comparison between experimental and control groups were performed by one-way ANOVA following Dunnett’s test (P <0.05, vs. control). The SPSS software (version 20; IBM Corporation, New York, USA) and Graph Pad Prism software (version 5; San Diego, California, USA) were used for the analysis and graph generation of all data, respectively. The obtained results are compared with the vehicle control group where the significance is presented at the level of P<0.05.
Effect of MEHL on thrombosis
The result of in-vitro antithrombotic activity of MEHL is shown in Table 1. The percent inhibition of thrombosis by the MEHL was 31.67±1.33 %, and 38.00±2.52 % at the dose of 100 and 200mg/mL, respectively. The percentage (%) of clot lysis was significant (P<0.05) when compared with control. The highest thrombolytic activity generated by MEHL at a dose of 200mg/mL showed potent effect of thrombolysis compared to the streptokinase's (standard) clot lysis (45.33±2.67 %) activity. On the other hand, distilled water treated as negative control exhibited a negligible percentage of lysis of clot (3.33±0.33 %). Result of clot lysis obtained after treating the clots with different dosages of crude extract (MEHL) is illustrated in Figure 1.

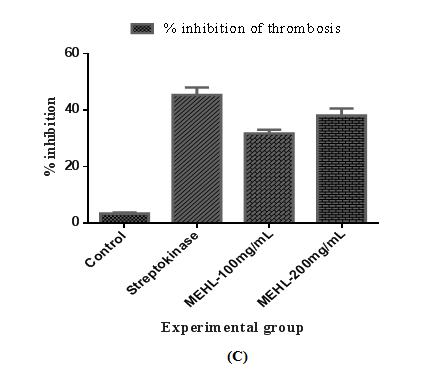
Figure 1 Effect of MEHL on thrombosis in in-vitro antithrombotic test.
Effect of methanolic extract of Euphorbia hirta leaves in the treatment on thrombosis. Number of human (n) =3. (A) Each bar represents the mean ± standard error of the mean of clot weight before lysis. (B) Each bar represents the mean ± standard error of the mean of clot weight after lysis. (C) Each bar represents the percentage (%) inhibition of thrombosis by respective groups. p<0.05 are compared to the values of the negative control group.
| Group Dose n Clot weight Clot weight Inhibition (%) |
| before lysis (gm.) after lysis (gm.) |
| Control 100µL 3 0.57±0.03 0.53±0.03 3.33±0.33 |
| Streptokinase 100µL 3 0.56±0.04 0.11±0.01 45.33±2.67 |
| MEHL 100mg/mL 3 0.54±0.03 0.22±0.02 31.67±1.33 |
| MEHL 200mg/mL 3 0.52±0.03 0.14±0.01 38.00±2.52 |
Table 1 Effect of MEHL on thrombosis in in-vitro antithrombotic test
All of the experimental values are presented as mean ± SEM (n=3), MEHL=Methanol extract of Euphorbia hirta leaves. p<0.05 vs. control group (Dunnett’s test).
Effect of MEHL on acute oral toxicity
No sign of toxicity, behavioral changes or mortality was observed upto the dose of 3200mg/kg for MEHL or control group. During the 14-day observation period, there was no change in food intake or other behaviors and was the same as prior to the experiment. So, the MEHL was safe upto a dose of 3200mg/kg body weight. Based on acute oral toxicity results, three different dosages (200, 400 and 800mg/kg) were selected for in vivo anti-inflammatory study.
Effect of MEHL on xylene-induced ear edema
The methanolic extract of leaves of Euphorbia hirta reduced the edema in the mice ear induced by xylene. The result of anti-inflammatory activity of crude extracts obtained from xylene-induced ear edema in mice is displayed in Table 2. It was observed from results of percentage inhibition of ear edema that all dosages of MEHL have a very significant impact on the reduction of ear edema in mice when compared to the control and was dose-dependent in manner. Among them, 51.06±6.86 % is the highest value of reduction that was generated by MEHL 800mg/kg, where the reduction value of standard is (58.78±2.90 %). Figure 2 clearly illustrates the observation of xylene-induced ear edema in mice.

Figure 2 Effect of MEHL on ear edema in xylene-induced ear edema in mice.
Effect of methanolic extract of Euphorbia hirta leaves treatment on xylene-induced ear edema in mice. Number of animals (n) =5; Difference between the weight of xylene-treated ear (right ear) and the weight of untreated ear (left ear) was considered to be the edema weight. (A) Each bar represents the mean ± standard error of the mean of ear edema weight of five animals. (B) Each bar represents the percentage (%) inhibition of edema formation by respective groups. p<0.05 are compared to the values of the negative control group.
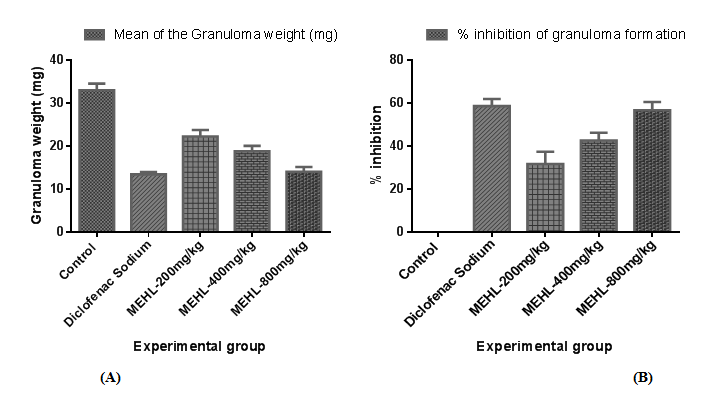
Figure 3 Effect of MEHL on ear edema in xylene-induced ear edema in mice.
Effect of methanolic extract of Euphorbia hirta leaves treatment on xylene-induced ear edema in mice. Number of animals (n) =5; Difference between the weight of xylene-treated ear (right ear) and the weight of untreated ear (left ear) was considered to be the edema weight. (A) Each bar represents the mean ± standard error of the mean of ear edema weight of five animals. (B) Each bar represents the percentage (%) inhibition of edema formation by respective groups. p<0.05 are compared to the values of the negative control group.
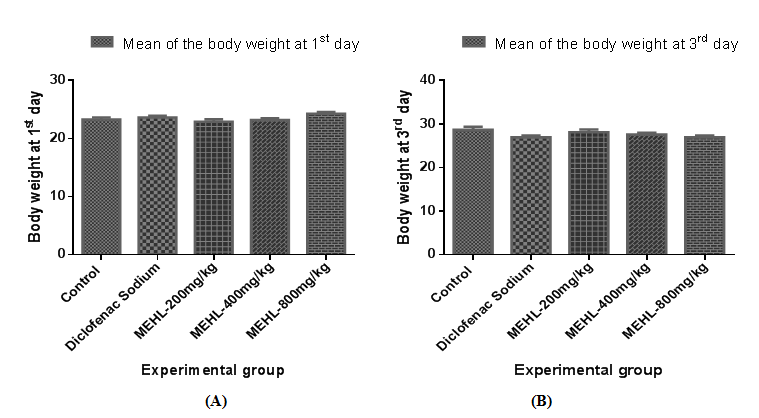

Figure 4 Effect of MEHL on ear edema in xylene-induced ear edema in mice.
Effect of methanolic extract of Euphorbia hirta leaves treatment on xylene-induced ear edema in mice. Number of animals (n) =5; Difference between the weight of xylene-treated ear (right ear) and the weight of untreated ear (left ear) was considered to be the edema weight. (A) Each bar represents the mean ± standard error of the mean of ear edema weight of five animals. (B) Each bar represents the percentage (%) inhibition of edema formation by respective groups. p<0.05 are compared to the values of the negative control group.
Group Dose n Ear weight differences (mg) Inhibition (%) |
Control 10mL/kg 5 10.45±0.61 0.00±0.00 |
Table 2 Effect of MEHL on ear edema in xylene-induced ear edema in mice
All of the experimental values are presented as mean ± SEM (n=5), MEHL=Methanol extract of Euphorbia hirta leaves. p<0.05 vs. control group (Dunnett’s test).
Effect of MEHL on cotton pellet-induced granuloma formation
The cotton pellet-induced granuloma formation in mice was conducted to determine the therapeutic effect of MEHL to inhibit the chronic inflammation. The result of chronic anti-inflammatory activity of crude extracts obtained from cotton pellet-induced granuloma formation in mice is shown in Tables 3 & 4. All of the groups showed a dose-dependent, significant (∗𝑃<0.05 versus control) inhibition of granuloma formation in mice when compared to the control. Among them, MEHL at dose of 800mg/kg provided highest inhibition value (56.80±3.85 %) of granuloma formation in mice, where the inhibition value of standard is (58.69±3.32 %). In addition, MEHL extract as well as diclofenac sodium significantly decreased the body weight gain of animals. Figures 3 & 4 clearly illustrates both observations of cotton pellet-induced granuloma formation in mice.
Group Dose n Granuloma weight (mg) Inhibition (%) |
Control 10mL/kg 5 33.05±1.52 0.00±0.00 |
Table 3 Effect of MEHL on granuloma formation in cotton pellet-induced granuloma formation in mice
All of the experimental values are presented as mean ± SEM (n=5), MEHL=Methanol extract of Euphorbia hirta leaves. p<0.05 vs. control group (Dunnett’s test).
Group Dose Weight at 1st day Weight at 3rd day Weight at 8th day |
Control 10mL/kg 23.30±0.34 28.70±0.67 28.28±0.65 |
Table 4 Effect of MEHL on body weight in cotton pellet-induced granuloma formation in mice
All of the experimental values are presented as mean ± SEM (n=5), MEHL=Methanol extract of Euphorbia hirta leaves.
As a significant reservoir of natural remedies, plant-derived medicines have a trustworthy long history of use for the preclusion and treatment of human diseases and potentially helpful for the discovery and development of fancy compounds leading to drugs.2,28 The results of our study clearly demonstrate that MEHL significantly represents in vitro thrombolytic and in vivo anti-inflammatory activities though these therapeutic responses were statistically lower than reference drugs.
Pharmacologically, the acute oral toxicity study in mice is a crucial factor in case of search the therapeutic index of medicines and xenobiotics and to select the rational administrative dosages that are safe, non-toxic and most effective for patients.29 Up to the dose as high as 3200mg/kg in experimental condition no mortality was observed and thus LD50 of Euphorbia hirta leaves extract could not be obtained. The crude extract was found to be safe and non-toxic with a broad therapeutic range and three comparatively high doses (200mg/kg 400mg/kg and 800mg/kg) were used for in-vivo doses.
Nevertheless the comprehensive knowledge acquired so far about antithrombotic activity of Euphorbia hirta leaf extract, this is the first time an antithrombotic effect of Euphorbia hirta leaf extract is determined in vitro. By activating the enzyme plasminogen and converting into plasmin most thrombolytic agents exert their therapeutic effect, which degrades many blood plasma proteins, including fibrin clots.30,31 It is evidenced that cell surface-bound bacterial plasminogen is easily converted into the active form plasmin, which could lead to fibrinolysis.30 Therefore, lysis of clots is essential for the management of clot-related disorders, including myocardial infarction (heart attack), thromboembolic strokes, arterial thromboembolism, deep vein thrombosis, and pulmonary embolism, to clear a blocked artery that prevents permanent damage to the respective tissues.32 Streptokinase (SK), a potent plasminogen activator is secreted from the several species of streptococci and works as cofactor molecule that binds with both plasminogen and fibrinogen. Streptokinase activates plasminogen to lysis fibrin clots and also destroys the extracellular matrix and fibrin fibers that hold cells together despite the presence of the major physiological plasmin inhibitor, α2-antiplasmin.33 Streptokinase has been used to treat acute myocardial infarction in developing countries, though plasminogen activator is widely used to manage the disease in developed countries.34 In this assay, Euphorbia hirta leaf methanolic extract at both dosages (100 & 200mg/mL) exhibits the moderate antithrombotic activity. Here, MEHL 200mg/mL elicited relatively maximum clot dissolution properties and the percentage inhibition is (38.00±2.52), whereas, reference drug streptokinase exhibits (45.33±2.67%) inhibition. The augmentation in cot lysis by MEHL compared to the negative controls portrays its significant thrombolytic activity and scientifically supports its potential use in clot-related disorders.
In this study, two tests were employed for evaluating the anti-inflammatory activity of methanolic leaf extract of Euphorbia hirta, i.e., xylene-induced ear edema and cotton pellet-induced granuloma formation. Xylene, the inflammatory agent was applied to estimate the probable anti-inflammatory effectiveness of the plant extract under study. Xylene-induced ear edema model is one of the most workable and extensively used procedures to elucidate the possible topical anti-inflammatory activity of a drug and its probable action mechanism, in case of acute phase of inflammation.35 In acute phase of inflammation, in order to provoke vasodilatation and vascular permeability as well as edema four key inflammatory mediators, namely histamine, serotonin, bradykinin, and prostaglandin (PG) are released.35,36 On the other hand, xylene-induced ear swelling is accompanied by innate immunity response of the skin, a cytotoxicity reaction of activated T cells and then migration of PMN leucocytes which augment swelling and heaviness of the ear.37 Pharmacologically, the inhibition of fluid accumulation generated by topical use of xylene at the treatment site is considered as anti-inflammatory activity.38 The methanolic extract of Euphorbia hirta leaves significantly inhibited the ear edema formation in mice induced by xylene due to hindrance of phospholipase A2, which is the precursor of all inflammatory activity.35 Respective plant extract shows their antiedematous effect by significantly reduced ear swelling in mice. This result suggested that MEHL (200, 400 and 800mg/kg, orally) possessed anti-inflammatory activity by inhibition of fluid accumulation through blocking the inflammatory mediators of the acute phase of inflammation. Here, MEHL 800mg/kg elicited the strongest anti-inflammatory effect (51.06±6.86 %) against xylene-induced topical acute inflammation, whereas, the standard drug showed (58.78±2.90 %) percentage of inhibition. So, further scientific study is required to elucidate the exact mechanism by which the plant extract inhibited ear swelling.
In case of chronic phase of inflammation, the cotton pellet-induced granuloma model has been widely used to screen the possible anti-inflammatory activity of a compound and its probable action mechanism.39 It is well known that the synthesis and release of inflammatory cells including neutrophils, macrophages, and fibroblasts are responsible for granuloma formation in the cotton pellet-induced granuloma model.27 In addition, the host inflammatory response and the modulation of release of inflammatory mediators are triggered by subcutaneously implanted material (cotton pellet), which finally lead to tissue proliferation and produces granuloma in mice.40 Transudative, exudative, and proliferative are the three response phases of the subcutaneous implantation of cotton pellet in mice. Leakage of fluid from blood vessels triggered by increasing in vascular permeability is regarded as transudative phase, the first phase of response. The leakage of protein from bloodstream around granuloma triggered by the intensive maintenance in vascular permeability change is called the exudative phase, the second or intermediate phase of response. Proliferative phase is the third or final phase and defined as the generation of granulomatous tissues caused by continuous release of pro-inflammatory mediators.27,41 Strong inhibition in granuloma formation is exerted by steroidal anti-inflammatory drugs on both the transudative and proliferative phases of inflammation, whereas diclofenac sodium, one of the NSAIDs drug, provide inhibitory activity only in the final or third phase (proliferative) of inflammation, by inhibiting the cyclooxygenase accompanied with prostaglandin synthesis.27 In this study, the methanol extract of Euphorbia hirta leaves decreased the dry weight of the cotton pellets compared to control groups. The experimental groups treated with plant extract (MEHL 200, 400 and 800mg/kg, orally) elicited a noticeable inhibition on granuloma formation as well as significantly decreased the body weight gain. Among three dosages, MEHL 800mg/kg showed the highest percentage inhibition (56.80±3.85) of granuloma formation in mice and approximately closed to the standard (58.69±3.32) group.
Therefore, the existence of flavonoids, tannins, triterpenoids and glycosides in MEHL 19 may be responsible for their significant anti-inflammatory effect 8 that was observed in both experiments. But further scientific study is needed to identify the specific active compounds and confirm the potential mechanism by which the plant extracts reduced inflammation.
Our experimental documents are in agreement with previous claims and demonstrate that methanolic extract of Euphorbia hirta leaves possesses the rich amount of anti-inflammatory properties, as shown by the inhibition of ear edema and granuloma formation in mice. Moreover, it could be effective interference in the thrombosis process. These outcomes strongly support the traditional uses of this plant scientifically, in case of thrombosis and inflammation management. However, further quantitative chemical investigation is needed to isolate and elucidate the structure of the active constituents involved in the thrombolytic and anti-inflammatory activities.
The authors are much grateful to the Department of Pharmacy, Jashore University of Science & Technology, Jashore, Bangladesh for providing facilities to carry out the research work smoothly.
This arduous research work did not have any particular funding. The total cost of completing the research work was carried by authors own finance.
None.
Authors hereby declare that “Principles of laboratory animal care” (NIH publication No. 85–23, revised 1985) were properly followed. All experimental design of this study were examined and approved by ethical research committee of Jashore University of Science and Technology, Jashore- 7408, Bangladesh.
Authors declared no conflicts of interest.

©2019 Rahman, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.