International Journal of
eISSN: 2381-1803


Research Article Volume 17 Issue 1
1Department of Food Science and Technology, Federal University of Technology, Akure, Nigeria
2Department of Food Technology and Biotechnology, Lagos State University of Science and Technology, Lagos, Nigeria
Correspondence: Ifesan BOT, Department of Food Science and Technology, Federal University of Technology, Nigeria
Received: December 26, 2023 | Published: February 6, 2024
Citation: Ibitoye AO, Oguntuase SO, Oguntoyinbo OO, et al. Antioxidant, phytochemical and fatty acid profile of extracts from bark of Pterocarpus erinaceus. Int J Complement Alt Med. 2024;17(1):17-24 DOI: 10.15406/ijcam.2024.17.00678
Pterocarpus erinaceus is reported locally to replenish the body nutrient, support the production of red blood cells and possesses anti-inflammatory activity. Standard methods were used to investigate the chemical constituents of the field and market bark samples of P. erinaceus. Field sample of P. erinaceus had significantly higher protein, (14.15%; 13.90%), than the market sample. The bark extracts had iron (4.04mg/100g-5.19mg/100g) and other minerals. Phytochemicals in P. erinaceus bark extracts include; flavonoid, saponin, phytate and tannin. The highest antioxidant activity was obtained from field bark water extract (FBW) with an IC50 of 12.53µg/ml. Fatty acid profile of P. erinaceus bark revealed that it contains linoleic acid (72.35%), palmitic acid (18.73%), D-limonene (44.93%) and D-carvone (11.99%). This study gathered that bark extracts of P. erinaceus contain essential nutrients and fatty acid which may support growth in the body and may be employed in the production of functional food.
Keywords: antioxidant, antibacterial, bark extract, fatty acids, phytochemical, Pterocarpus erinaceus
Plants have been recognized for their several lifesaving and therapeutic properties. Due to the increasing concerns about the sustainability of human living, good health and well-being, the control of damaging effects of microorganism and synthetic drugs is important and should be greatly encouraged.1
Pterocarpus erinaceus is distributed throughout the West and Central African Savannah and dry forest known as “Madabiya” in Hausa (Northern Nigeria) and “Apepe” “Ibo” or “Agbolosan” in Southwest Nigeria. A decoction of the stem bark issued as astringent for severe diarrhea or dysentery,2 anti-inflammatory,3 wound healing, restorative, tonic and as ingredient in abortifacient prescription.4 It is used traditionally for the dressing of ring worms on the scalp, soup making, treatment of laxation; pulmonary trouble, anemia5 and the stem bark demonstrated anti-ulcer activity.6 In addition, regular consumption of Pterocarpus erinaceus was found to exhibit healing effect on cardiovascular diseases, prostate, gastrointestinal and epithelial cell cancers,7 depression and alzhemier through its antioxidant properties.8
Pterocarpus angolensis bark paste, was blended together with garlic and was documented to increase the keeping quality of fresh catfish stored in the refrigerator.9 Pterocarpus osun L. extract has been shown to possess antibacterial activity against multi-drug resistant Escherichia coli isolated from wounds.10
Hence this research was carried out to determine the chemical, antioxidant, antibacterial, and fatty acids content of the bark of market and field samples of Pterocarpus erinaceus to further investigate the reports from the local consumers of the plant.
Collection of samples
Freshly harvested stem bark of Ptercarpus erinaceus plant (FB) was obtained from premises of Adekunle Ajasin University, Akungba- Akoko, Ondo State, Nigeria. It was identified at the Crop Soil and Pest Management Department, Federal University of Technology, Akure, Nigeria. The market bark sample of Ptercarpus erinaceus was purchased at local market in Akure, Ondo State, Nigeria.
Determination of proximate composition of P. erinaceus bark samples
Proximate composition of samples were done using the method of Association of Official Analytical Chemists.10 Parameters determined include moisture, protein, ash, fat, fibre and carbohydrate.
Determination of mineral content of bark samples of Pterocarpus erinaceus
Two grams of sample was placed in a crucible and left to ash in a muffle furnace at 550⁰C for 5 h after which it was transferred into the desiccator to cool. The ash samples were dissolved mixture of 1 ml nitric acid and 1 ml HCl and made up to 100 ml. This was used to assay for Magnesium, sodium, calcium, potassium, manganese, copper, zinc and iron using atomic absorption spectrophotometer. Flame photometer was used to determine Na and K while phosphorus was determined calorimetrically (AOAC, 2012).10
Preparation of ethanol and aqueous extracts from P. erinaceus bark
The bark was air-dried for 30 days and ground using a blender to obtain a fine powder used for extract preparation. Four hundred and fifty grams (450 g) of the field bark (FB) sample and the market bark (MB) sample were each cold macerated in 1350 ml of ethanol and 1359 ml of distilled water (1.3 w/v) for three days respectively. The mixture was stirred every 24 h and was sieved with mesh cloth and filtered with whatman paper no 1 on the 3rd day. The extract was there after concentrated with rotary evaporator at 50oC and kept at 4oC for further use.
Phytochemical analyses of bark extract from Pterocarpus erinaceus
Determination of saponin: Two grams of the ground sample was weighed into a beaker and 100 ml of n-butanoic acid was added. The mixture was shaken for 5 h then filtered with No1 Whatman filter paper into a beaker containing 20 ml of 40% saturated solution of magnesium carbonate (MgCO3). The mixture obtained was re-filtered through No 1 Whatman filter paper to obtain a clean clear solution. One millilitre of the solution was taken into volumetric flask while 2 ml of 5% iron (III) chloride (FeCl3) solution was added and made up to the mark with distilled water. This was allowed to stand for 30 min for the colour to develop while the absorbance was read against the blank at 380 nm.11
Determination of tannin composition: Sample (0.2g) was weighed and 10ml of 70% aqueous acetone was added, properly covered and were placed in an ice bath shaker for 2 h at 30⁰C. The solution was then centrifuged, supernatant was stored in ice, 0.2 ml was pipetted into the test tube and made up with 0.8 ml of distilled water. Standard tannic acid solutions were prepared and 0.5 ml of Folin Ciocateau reagent was added to both samples and standard followed by 2.5 ml of 20% Na2CO3. The solution was then vortex and allowed to incubate for 40 min at room temperature, its absorbance was read at 725 nm against a reagent blank.12
Determination of phytate composition: Four grams of sample was soaked with 100 ml of 2% HCl for 3 h and then filtered through a No 1 Whatman filter paper. Twenty five milliliter of the filtrate was taken inside a conical flask and 5 ml of 0.3% of ammonium thiocyanate solution was added as indicator. Distilled water (53.5ml) was added and titrated against 0.00566g/ml of standard iron (III) chloride solution that contained 0.00195 g of Fe/ml until a brownish yellow colouration persist for 5min.13
Estimation of total flavonoid contents of bark samples of Pterocarpus erinaceus: Sample (0.5ml) was mixed with 0.5 ml methanol, 50μl of 10% AlCl3, 50μl of 1mol l-1 potassium acetate and 1.4ml water, and incubated at room temperature for 30 min while the absorbance of the mixture was taken at 415nm. The total flavonoid was calculated using quercetin as standard and results were expressed as mg quercetin equivalents per gram of the sample.14
Evaluation of antioxidant properties of bark extracts from Pterocarpus erinaceus
Determination of total phenol content of bark samples: Total phenol content of P. erinaceus bark extract was determined by the Folin-Ciocalteu assay using the method.16 Five milliliter of distilled water with 0.25 ml extract was added to Folin-Ciocalteu’s phenol reagent. After 2min, 3.75ml of 20% (w/v) sodium carbonate solution was added. The mixture was left to stand for 2h after which the absorbance was measured at 760nm using a Lambda EZ150 spectrophotometer (Perkin Elmer, USA). The standard used was tannic acid and the results were expressed as mg tannic acid equivalent per gram of the sample.
Determination of free radical (DPPH) scavenging of bark extract: Extract (0.2ml) was added to 3.8ml of 0.004% methanolic DPPH solution. This was placed in the dark at room temperature for 60min and the absorbance was measured at 517 nm. A blank sample containing only methanol was used to zero the spectrophotometer. Ascorbic acid was used for comparison.15
Determination of ABTS radical scavenging activity: The ability of the extract to scavenge 2, 2’-azinobis-(3- ethylbenzothiazoline-6-sulfonic acid) was determined.16 The ABTS•+ (mother solution) was prepared by mixing equal volumes of 8 mM ABTS and 3mM potassium persulphate (K2S2O8), and allowed to react for 12h in a dark place. The working solution was prepared by mixing 5 ml of the mother solution with 145 ml phosphate buffer (pH 7.4). A range of trolox standard solutions (100–1000μM) were prepared in acidified methanol. The working solution (2.9ml) was added to the methanolic extracts (0.1ml) or Trolox standard (0.1ml) in a test tube, vortex and allowed to stand for 30 min. The absorbance of the standards and samples were measured at 734nm with a Lambda EZ150 spectrophotometer.
Determination of ferric reducing antioxidant power (FRAP) of extract: The reducing power of the extracts was determined by measuring the ability of the extract to reduce FeCl3 solution.17 Appropriate dilutions of the extract (2.5 ml) was mixed with 2.5 ml of 200 mM sodium phosphate buffer (pH 6.6) and 2.5 ml 1% potassium ferricyanide. The mixture was incubated at 50 oC for 20 min, 2.5 ml 10% trichloroacetic acid was added and the mixture was centrifuged at 353x g for 10 min. Five millilitre of the supernatant was mixed with an equal volume of water and 1 ml of 0.1% ferric chloride. The absorbance was measured at 700 nm.
Determination of cupric ion reducing antioxidant capacity (CUPRAC): Four hundred microliter of extract was mixed with1 ml of aqueous copper (II) chloride, 1 ml alcoholic neocuproine and 1ml ammonium acetate aqueous buffer at pH 7. The mixture was incubated at room temperature for 30min and the developed absorbance was read at 450nm
Determination of phenolic compounds in bark samples of Pterocarpus erinaceus
Phenolic compounds in bark extracts were estimated at 280nm by an Agilent 1100 series HPLC system (Agilent technologies, USA). Separation was carried out on a Zorbax XDB column (150mmx4.60mm). A gradient elution was performed with sterile water containing 1% formic acid and acetonitrile. The flow rate was 0.7ml/min and the injection volume was 5µl. Identification of the phenolic compounds was carried out by comparing their retention times to those of standards which include caffeic acid, p-coumaric acid and ferulic acid.
Antibacterial activity of crude extracts from Pterocarpus erinaceus bark
Bacteria used for assay (Escherichia coli and Staphylococcus aureus) were collected from Department of Microbiology Federal University of Technology Akure while Pseudomonas aeruginosa, and Moraxella catarrhalis obtained from State Specialist Hospital, Akure, Nigeria were each inoculated into the MacCartney bottles and incubated at 32⁰C for 24 h. The extracts were tested for activity against bacterial using modified agar-well diffusion method as described by Clinical and Laboratory Standard Institute.18 Five hour broth cultures of the test bacteria were adjusted to 108cfu/ml and applied on the surface of Nutrient agar (Hi Media Laboratories Limited, Mumbai, India). Sterilized Nutrient agar was poured into Petri dishes and allowed to solidify. Organisms were streaked over the solidified media surfaces. A sterile flamed cork borer of 8mm diameter size was used to punch four wells into each of the seeded plates and 0.6 ml of each extract was dispensed in each well. Controls were set up by filling wells with 1% of various solvents used. The plates were then incubated at 35°C for 24 h. The experiments were performed in duplicate and the means of the diameters of the inhibition zones were calculated.
Determination of fatty acid profile of Pterocarpus erinaceus bark samples
The analysis was performed using a Hewlett Packard 5890 II GC, equipped with a FID and HP-5 MS capillary column (30m· 0.25mm i.d., 0.25µm) and a HP 5972 mass selective detector. For GC–MS detection an electron ionization system with ionization energy of 70eV was used. Helium was the carrier gas, at a flow rate of 1ml/min. Injector and MS transfer line temperatures were set at 220 and 290⁰C, respectively. Column temperature was initially kept at 50⁰C for 3 min, then gradually increased to 150⁰C at a 3⁰C/min rate, held for 10 min and finally raised to 250⁰C at 10⁰C/min. Diluted samples (1/100 in acetone, v/v) of 1.0µl were injected manually and in the split less mode. The components were identified based on the comparison of their relative retention time and mass spectra with those of standards, NIST11 library data of the GC–MS system and literature data.
Proximate composition of P. erinaceus bark samples
The proximate composition of the field bark and the market bark samples is represented on Table 1. The fat content of the field bark and market bark were 14.92% and 13.56% respectively. The crude fibre content of FB was 23.80% and that of MB was 35.43%. The protein content of the field bark and market bark were 14.15% and 13.90% respectively. The ash content of FB was 9.97% and that of MB was 9.05%. The carbohydrate content of FB was 27.68% and that of MB was 19.93%. The field bark showed significantly higher concentration of protein, carbohydrate and fat than the market bark. Similar observations were made from the study on the nutritional content of the leaves of P. erinaceus.19 The differences may be attributed to processing methods, time of sample collection and source of sample. Different processing methods and storage time affects the physicochemical properties of plants and their overall nutritional value.20 The proximate result may agree with the claim by local users that P. erinaceus is a rich source of balanced nutrients needed for good health. Crude fibre content of P. erinaceus was higher compared with those obtained from the bark of African peach tree (2.07%) and Moringa oliefera stem bark (4.87%) as reported21 and respectively.22 Fibre prevents cholesterol from entering into the bloodstream, cleans the digestive tract and reduces the risk of coronary heart diseases. It adds bulk to food and helps in peristaltic movement of food. The bark is also rich in protein (14.15% for FB and 13.90% for MB) which is considerably higher than the concentration of protein in Moringa oliefera stem bark (1.33%).22 Protein can serve as a complement to carbohydrate and fat rich food. Protein is an essential component of food that is used in the body as enzymes for catalysis, building worn-out cells and tissue and in general metabolic regulation. Pterocarpus erinaceus bark is also a rich source of carbohydrate (19.93%-27.68%). The fat content of the bark is moderate for fat content expected from bark samples and similar to that of Moringa oliefera tree bark with fat content of 17.47%.23
|
Sample |
Moisture |
Fat |
Crude fibre |
Protein |
Ash |
CHO |
|
FB |
9.48±0.02b |
14.92±0.01b |
23.80±0.03a |
14.15±0.02b |
9.97±0.00b |
27.68±0.03b |
|
MB |
8.13±0.05a |
13.56±0.04a |
35.43±0.01b |
13.90±0.02a |
9.05±0.01a |
19.93±0.09a |
Table 1 Proximate composition (%) of bark samples of Pterocarpus erinaceus
Values are means±standard deviation of three determinations. Values with different superscripts along the columns are significantly different (p<0.05)
FB, field bark; MB, market bark
Mineral composition of P. erinaceus bark samples
Table 2 showed the mineral composition of P. erinaceus bark samples. Field bark (FB) had sodium content of 334.02 mg/100g and market bark (MB) of 362.14 mg/100g. Potassium present in FB was 7504.53mg/100g and MB was 6945.13 mg/100g. Potassium helps in protein synthesis from amino acids and in blood and the body tissues. Sodium to potassium ratio in the body is of great importance for prevention of high blood pressure, and Na/K ratio less than one is recommended.24 The Na/K ratio for the bark samples are less than one. This may mean that P. erinaceus could be employed in the maintenance of blood pressure. Calcium content of the bark samples (2175.53mg/100g for FB and 2237.56mg/100g for MB) are considerably higher than those reported for bark of African pear (832.40mg/100g) and bark of Mango tree (116.6mg/100g).24 Calcium is endeared as a key player in bone formation and other physiologic systems in the body. It is a mineral that is also implicated in vitamin D activities in the body. The sample had high phosphorus content of 2412.54mg/100g and 2412.54mg/100g for FB and MB respectively which are similar to that obtained from Morinda lucida (2401.60 mg/100g) and slightly lower than that observed for the bark of mango tree (3272.50mg/100g).25 Calcium and phosphorus are the main minerals present in the largest quantity in the structure of the body and bones.26 This may imply that consumption of P. erinaceus bark may offer support in the formation of strong bones and healthy teeth. Pterocarpus erinaceus bark was found to be rich in magnesium (455.82mg/100g and 299.05mg/100g) and the values are higher than those observed for other plant barks such as cashew tree bark (214.80mg/100g) and mango tree bark (304.10 mg/100g).24 Magnesium acts as activator of some enzyme systems and maintains the electrical potential in body nerves.27 The result from this work showed that zinc (2.38mg/100g and 2.05mg/100g) was detected in the FB and MB samples. Zinc is vital for the physiologic functions of living tissues and, regulation of many biochemical processes in the body.28 Zinc is notable for the production of insulin29 and can be involved in the prevention and management of diarrhoea. Local diets available for all ages especially the pregnant and lactating mothers are generally deficient in zinc.30
|
Minerals |
Field bark |
Market bark |
|
Sodium |
334.02±0.05a |
362.14±0.06b |
|
Potassium |
7504.53±0.13b |
6945.13±0.21a |
|
Calcium |
2175.53±0.06a |
2237.56±0.09b |
|
Phosphorus |
2289.39±0.05a |
2412.54±0.02b |
|
Magnesium |
455.82±0.03b |
299.05±0.07a |
|
Iron |
4.04±0.00a |
5.19±0.01b |
|
Zinc |
2.38±0.04b |
2.05±0.01a |
|
Nickel |
0.48±0.02b |
0.36±0.00a |
|
Cadmium Lead |
0.12±0.01b ND |
0.00±0.00a ND |
Table 2 Mineral composition (mg/100g) of bark samples of Pterocarpus erinaceus
Values are means ±standard deviation of three determinations. Values with different superscripts in the same row are significantly different (p<0.05).
Where; FB = Field Bark, MB = Market Bark, ND = Not detected
Phytochemical content of Pterocarpus erinaceus bark extracts
Table 3 shows the phytochemical content of P. erinaceus bark. It was found that saponin, phytate, tannin and flavonoids are present. Phytochemicals in P. erinaceus stem bark was also reported31 with the absence of steroids.19 Terpenoid was found in the aqueous and methanolic bark extracts of P. erinaceus.31 Differences in phytochemical components of plants may be as a result of genetic variations and environmental conditions. The field bark (FB) is a better source of phytochemical than the market bark (MB). The saponin content was from 31.39mg/g - 58.75mg/g with the highest value from FBW. The presence of saponin in the bark sample may support its use in controlling human cardiovascular disease and reduce blood cholesterol.32 Saponins contributed to the health and proper functioning of the immune system by binding to germs and other pathogens. Saponin can be referred to as a natural antibody.33 Tannin content of bark samples ranged from 24.17mg/g - 69.58mg/g while water extracts were richer in tannin than ethanol extracts. Tannin has antibacterial, anti-enzymatic and astringent properties. Ingestion of tannin causes constipation and can be used in the treatment of diarrhea.36,4 This may corroborate the report by local users that P. erinaceus is employed in the treatment of diarrhoea. Phytate content of P. erinaceus (4.21mg/g to 8.93mg/g) with the highest in market bark ethanolic extract and the lowest in Field bark water extract. Phytic acid may reduce occurence of colon cancer and prevent other inflammatory bowel disease. The low phytate level from the bark may not have any adverse effect on digestibility as when present in high concentration.35 Phytic acid has been reported to act as an antioxidant by binding minerals in the gut and prevent the formation of free radicals. In addition, it binds heavy metals such as cadmium and lead and hinders their accumulation in the body.36 Flavonoid content in the bark samples ranged from 2.63mg/g - 39.60mg/g with the highest value in water extract from field bark and the lowest in ethanolic extract of market sample. Flavonoids are free radical scavengers that prevent oxidative damage and possess strong anti-cancer activity. They also offer protection against allergies, inflammation, ulcer and tumor.37 The presence of phytochemical in P. erinaceus stem bark may suggest why the bark is employed in the treatment of several diseases by the local users.
|
Sample |
Tannin |
Flavonoid |
Saponin |
Phytate |
|
FBE |
33.75±0.00b |
4.21±0.00b |
47.38±0.03c |
6.01±0.02c |
|
MBE |
24.17±0.01a |
2.63±0.01a |
41.47±0.01b |
4.21±0.00a |
|
FBW |
69.58±0.00d |
39.60±0.01d |
58.75±0.02d |
8.93±0.04d |
|
MBW |
46.67±0.00c |
4.40±0.00c |
31.39±0.01a |
4.72±0.02b |
Table 3 Phytochemical composition (mg/g) of P. erinaceus bark extracts
Values are means ±standard deviation of three determinations. Values with different superscripts along the columns are significantly different (p<0.05)
FBE, field bark ethanol extract; MBE, market bark ethanol extract; FBW, field bark water extract; MBW, market bark water extract
Antioxidant properties of the bark extracts of P. erinaceus
The total phenolic content of the crude extracts is shown on Figure 1. Field bark water extract (FBW) yielded the highest phenol content (215.44 GAE mg/g) followed by market bark water extract (MBW) with phenolic content of 49.65 GAE mg/g of extract. Field bark ethanol extract (FBE) had total phenolic content of 39.65 GAE mg/g and market bark ethanol extract (MBE) had phenolic content of 27.193GAE mg/g of extract. The results showed that the water extracts had higher total phenolic content than the ethanol extracts. This might be due to the presence of water soluble phenolics in Pterocarpus erinaceus bark.
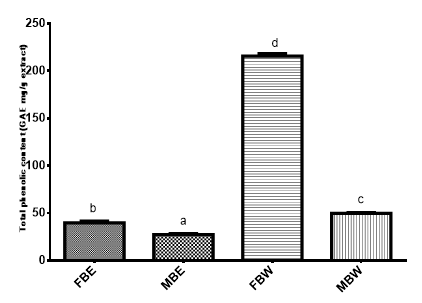
Figure 1 Total phenolic content (GAE mg/g) of P. erinaceus bark extracts.
Bars with different superscripts are significantly different (P<0.05)
FBE, field bark ethanol extract; MBE, Market Bark Ethanol Extract; FBW, field bark water extract, MBW, market bark water extract
The results of the phenolic compounds in P. erinaceus bark are shown in Table 4. The phenolic compounds detected were caffeic acid, p-Coumaric acid and ferulic acid. The field bark extracts had higher activity than their market bark counterparts. p-Coumaric acid is a phenolic compound with molecular formular of C9H8O3 (m/z 164) found in a wide variety of edible plants, may reduce the risk of stomach cancer and possess antioxidant properties.38 p-Coumaric acid found in honey exhibited in vitro anti-inflammatory activity.39 Ferulic acid [C10H10O4 (m/z 194)] is an abundant phenolic compound in plant cell walls used in cosmetic because of its antioxidant properties. Caffeic acid has a variety of potential pharmacological effects in in vitro studies and in animal models. Furthermore the inhibitory effect of caffeic acid on cancer cell proliferation by an oxidative mechanism in the human HT-1080 fibrosarcoma cell line has been established.40 Anti-inflammatory, anti-mutagenic, anti-bacterial and anti-carcinogenic properties demonstrated by caffeic acid has been attributed to its antioxidant properties.41 Phenolic compounds are a class of antioxidant agents which act as free radical scavengers, function in retardation of oxidative degradation of lipids with reduced risk of chronic diseases.42 There is a strong relationship between phenolic content and antioxidant activity of selected fruit and vegetable.43 Availability of phenolic compounds in P. erinaceus bark extracts may be responsible for its nutritional and health potential.
|
|
Concentration (ppm) |
|
|
Compound |
Field bark |
Market bark |
|
Caffeic acid |
21.56 |
0.078 |
|
p-coumaric acid |
0.038 |
0.017 |
|
Ferulic acid |
1.13 |
0.048 |
Table 4 Phenolic compounds of ethanolic extracts of Pterocarpus erinaceus bark
The total flavonoid content of FBW extract was 39.60 QE mg/g being the highest followed by MBW (4.40 QE mg/g), then FBE (4.21 QE mg/g) and MBE (0.62 QE mg/g) as shown on Figure 2. The total flavonoid content also revealed the same trend as total phenolic content The aqueous extracts (FBW and MBW) have significantly higher TFC than the ethanol extracts (FBE and MBE). Also, the field bark extracts had higher activity than the market bark extracts. Flavonoids perform antioxidant activity using scavenging or chelating process playing a preventive role in cancer and heart diseases.44
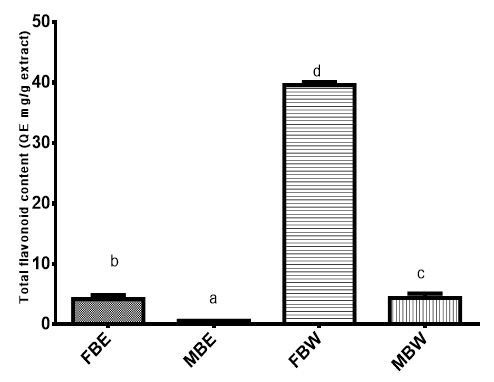
Figure 2 Total flavonoid content (QE mg/g) of P. erinaceus bark extracts.
Bars with different superscripts are significantly different (P<0.05)
FBE, field bark ethanol extract; MBE, market bark ethanol extract; FBW, field bark water extract; MBW, market bark water extract
The cupric reducing antioxidant capacity (CUPRAC) of the bark extract is shown on Figure 3. The capacity of FBW was the highest with 153.30TE mg/g followed by MBW at 34.44TE mg/g then FBE at 23.68TE mg/g and 17.11TE mg/g for MBE.
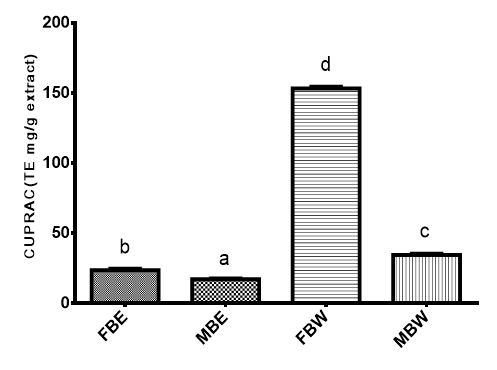
Figure 3 Cupric ion reducing antioxidant capacity (CUPRAC) (mg/g) of Pterocarpus erinaceus bark extracts FBE, field bark ethanol extract; MBE, market bark ethanol extract; FBW, field bark water extract; MBW, market bark water extract
Figure 4 showed the DPPH+ radical scavenging ability of P. erinaceus bark extracts expressed in IC50. It was observed that FBW had the highest activity with an IC50 of 31.42µg/ml followed by MBW with an IC50 of 40.66µg/ml. FBE had an IC50 of 84.54 µg/ml and MBE an IC50 of 120.90µg/ml. This result revealed that the free radical scavenging activity demonstrated by P. erinaceus bark extracts was more than that of ascorbic acid (IC50 of 113.54µg/ml) which was used as standard.
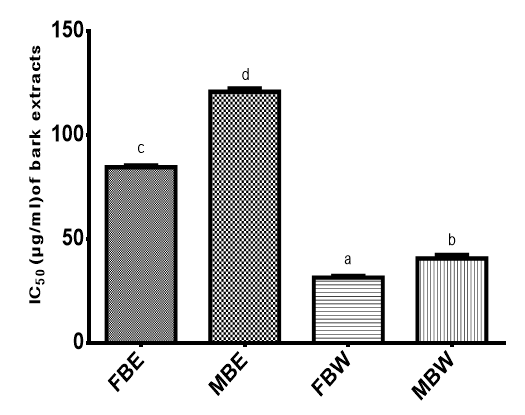
Figure 4 Free radical scavenging (DPPH+) ability of Pterocarpus erinaceus bark extracts expressed in IC50 (µg/ml).
Bars with different superscripts are significantly different (P<0.05)
FBE, field bark ethanol extract; MBE, market bark ethanol extract; FBW, field bark water extract; MBW, market bark water extract
The ability of the crude extract to scavenge radicals (ABTS+) is shown on Figure 5. The IC50 for FBW was 12.53µg/ml, 23.46µg/ml for MBW, 57.57µg/ml for FBE, 87.97µg/ml for MBE while trolox used as standard had an IC50 of 100.82µg/ml. Fifty percent and above inhibition of DPPH or ABTS radical is considered as significant for scavenging activity.45
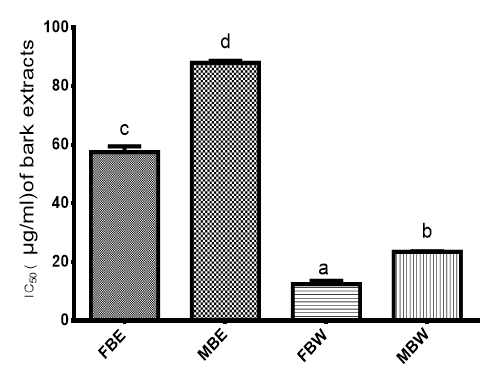
Figure 5 ABTS+ Radical scavenging ability of Pterocarpus erinaceus bark extracts expressed in IC50 (µg/ml).
Bars with different superscripts are significantly different (P<0.05)
FBE= Field Bark Ethanol Extract, MBE= Market Bark Ethanol Extract, FBW= Field Bark Water Extract, MBW= Market Bark Water Extract
Ferric reducing antioxidant power (FRAP) of the extracts is shown on Figure 6. The reducing power ranged from 0.0173mg/ml to 0.2883mg/ml. Field bark water extract had the highest reducing power of 0.2883mg/ml followed by MBW with 0.0437mg/ml then FBE with 0.0280mg/ml and MBE with 0.0173 mg/ml. The same trend was observed for CUPRAC where water extracts produced better scavenging properties than ethanol extracts.
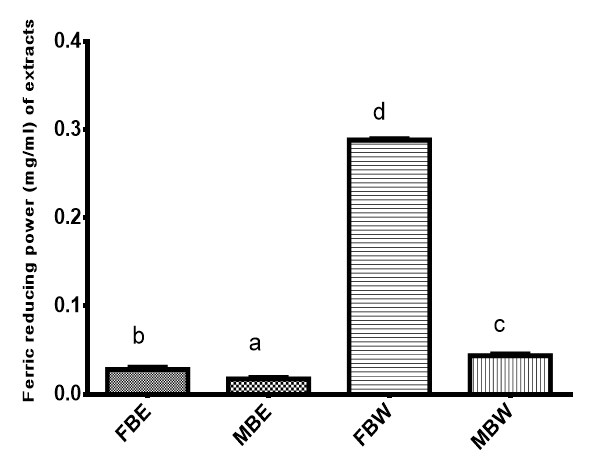
Figure 6 Ferric reducing antioxidant power (mg/ml) of Pterocarpus erinaceus bark.
Bars with different superscripts are significantly different (P<0.05)
FBE, field bark ethanol extract; MBE, market bark ethanol extract; FBW, field bark water extract; MBW, market bark water extract
Antibacterial properties of P. erinaceus bark
It was observed that the four extracts at the concentration used (1mg/ml) were unable to inhibit the four pathogenic bacteria tested which include; Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Moraxella catarrhalis. However, bio-guided fractionation of extracts of P. erinaceus plant parts at very low concentrations was reported to yield antibacterial activities against some human pathogens.46
Fatty acid /essential oil profile of bark samples of P. erinaceus
The fatty acid /essential oil profile of the field bark of P. erinaceusi is shown on Table 5. It revealed that the bark contains 1,2-benzenedicarboxylic acid, bis (2-methylpropyl) ester, n – Hexadecanoic acid (palmitic acid), 9 - Octadecanoic (Z)-methyl ester (methyl oleate), 9, 12-Octadecadienoic acid (Z, Z) (linoleic acid) and Bis (2-ethylhexyl) phthalate. The highest fatty acid found in the bark was linoleic acid (72.35%) while the lowest was methyl oleate (2.93%). Linoleic acid possesses anti-inflammatory and cancer preventive characters.47 It is an essential fatty acid that cannot be synthesized by the body and needs to be consumed from other sources. The compound, 1,2-benzenedicarboxylic acid, bis (2-methylpropyl) ester constitutes 2.93% of the oil and used in the preparation of perfumes and cosmetics.47 Palmitic acid consisting 18.73% of the oil and it is a fatty acid that is found naturally in animals and plants. Medicated oils rich in n-Hexadecanoic acid are used in the treatment of rheumatism and inflammation in India.48 Methyl oleate constitutes 2.90% of the oil. It is known to have anti-inflammatory, antiandrogenic, and an emiagenic property.49
|
Peak no |
Retention time |
Components |
Composition (%) |
|
1 |
34.466 |
1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester |
2.93 |
|
2 |
34.706 |
n - Hexadecanoic acid [palmitic acid] |
18.73 |
|
3 |
37.470 |
9 - Octadecanoic (Z) - , methyl ester [methyl oleate] |
2.90 |
|
4 |
38.271 |
9, 12-Octadecadienoic acid (Z, Z) [linoleic acid] |
72.35 |
|
5 |
45.086 |
Bis (2-ethylhexyl) phthalate |
3.09 |
Table 5 Fatty acid / essential oil profile of field bark sample of Pterocarpus erinaceus
The fatty acid /essential oil profile of the market sample of P. erinaceus bark is shown on Table 6 with eight compounds which include; butanoic acid, 3-methylbutyl ester (4.68%), D – Limonene (44.93%), 1, 6 – Octadien-3-ol or linalool (6.46%), 2-Cyclohexen-1-ol, 2-methyl- 5- (1-methylethenyl) -, cis-, also known as cis Carveol (4.99%), D- carvone (11.99%), Hexadecanoic acid, methyl ester or methyl palmitate (6.06%), 9 – Octadecenoic acid (Z) -, methyl ester (5.62%) and Phthalic acid, di (2-propylpentyl) ester (15.27%). Butanoic acid, 3-methylbutyl ester or Isoamyl butyrate is used as a flavour agent and in perfume production because of its sweet, chocolate like aroma. Limonene is a cyclic terpene and it possesses a strong smell of oranges. It is used in cosmetic products as a fragrance. It possesses antibactericidal effect and demonstrated a potential in food preservation.50 Linalool is known to possess anti-inflammatory activity.51 Cis- carveol is produced when limonene is oxidized in moist air. D- carvone is a member of the family of terpenoids found naturally in many essential oils and it is used in the food and flavor industry, aromatherapy and alternative medicine.52 Methyl palmitate has been found to possess a potent antifibrotic effect.53 Phthalic acid, di (2-propylpentyl) ester is known to possess cytotoxic effect.54
|
Peak no |
Retention time |
Components |
Composition (%) |
|
|
1 |
3.796 |
Butanoic acid, 3-methylbutyl ester [Isoamyl butyrate] |
4.68 |
|
|
2 |
5.513 |
D – Limonene |
44.93 |
|
|
3 |
7.264 |
1,6 – Octadien-3-ol [Linalool] |
6.46 |
|
|
4 |
10.325 |
2- Cyclohexene-1-ol, 2-methyl- 5- (1-methylethenyl) -, cis-[cis- Carveol] |
4.99 |
|
|
5 |
10.966 |
D-carvone |
11.99 |
|
|
6 |
32.131 |
Hexadecanoic acid, methyl ester [methyl palmitate] |
6.06 |
|
|
7 |
36.183 |
9 – Octadecenoic acid (Z) -, methyl ester |
5.62 |
|
|
8 |
46.122 |
Phthalic acid, di (2-propylpentyl) ester |
15.27 |
|
Table 6 Fatty acid / essential oil profile of market bark sample of Pterocarpus erinaceus
This study shows that the bark of P. erinaceus is rich in essential nutrients that is needed by the body such as crude fibre, protein, carbohydrate, mineral and fatty acids. Pterocarpus erinaceus bark also contains phytochemicals which may be responsible for the antioxidant properties exhibited and the local medicinal applications reported by the local consumers.
Beatrice O.T. Ifesan: Conceptualization, Supervision, Review and editing. Abidemi. O. Ibitoye: Methodology, Writing original draft. Oladotun. O. Oguntoyinbo: Editing. Babawande A. Origbemisoye: Review and editing.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
None.

©2024 Ibitoye, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.