International Journal of
eISSN: 2381-1803


Research Article Volume 17 Issue 2
1 Department of Nutrition, Exercise and Sports, University of Copenhagen, Denmark
2 Skejby University Hospital, Department of Woman and Child, University of Aarhus, Denmark
3 Institute for Clinical Research and Education, Frederiksberg, Denmark
4 Affiliated to Parker Institute, Frederiksberg Hospital, Nordre Fasanvej 57, 2000 Frederiksberg; Denmark
5 Preventive and Clinical Nutrition, Section for Nutrition and Health, University of Copenhagen, Denmark
6 Center for Translational Cardiology and Pragmatic Randomized Trials, Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark
Correspondence: Kaj Winther, Professor, MD, DMSci, Department of Nutrition, Exercise and Sports, Rolighedsvej 26, 1958 Frederiksberg C, University of Copenhagen, Denmark
Received: March 19, 2024 | Published: April 9, 2024
Citation: Winther K, Pedersen FH, Hansen PW, et al. A double-blinded, randomized, parallel grouped, phase III comparative study of Japanese White Turmeric extract and placebo in patients with mild to moderate osteoarthritis of the knee and or hip. Int J Complement Alt Med. 2024;17(2):81-92. DOI: 10.15406/ijcam.2024.17.00687
Background: Osteoarthritis is a common disease among middle aged and elderly people and paracetamol and NSAIDs, which can both cause side effects, are often used to manage symptoms like pain. This study aimed to test if Japanese White Turmeric (JWT), a new variant of turmeric, containing labdane terpenoid and hardly any curcumin, would lessen symptoms from osteoarthritis and reduce the consumption of rescue medication, without causing side effects.
Methods: Volunteers (n=120) with osteoarthritis of the knee and/or hip were randomly allocated to either daily treatment with JWT (12.8 mg) or placebo for 6 months. Primary effect variables, pain and physical function and secondary effect variables, stiffness and global severity of the disease (PGAD) were scored on WOMAC questionnaires initially and after 1, 2, 3 and 6 months of treatment. Rescue medication taken by participants were self-registered in a diary.
Results: JWT treatment showed a statistically significant reduction in WOMAC pain after 1, 2 and 3 months (p<0.0003). After 6 months this decline was still statistically significant and superior to placebo (p<0.041). Effect size was 4.961±2.366 (n=40) for treatment group vs (n=27) for placebo), 95% confidence interval was 9.686 to -0.2359, p< 0.039. An identical pattern was observed when testing physical function, joint stiffness and PGAD. The consumption of paracetamol was significantly lowered after 3 and 6 months of active treatment (p<0.014 and p<0.050) respectively vs placebo. A similar pattern was observed for NSAIDs. No serious side effects were reported and minor side effects were equally represented in both groups.
Conclusion: Our data suggests that the herbal remedy, Japanese White Turmeric significantly alleviates symptoms of osteoarthritis, including pain and lower the consumption of rescue medication.
Keywords: pain, daily activity, paracetamol, NSAIDs, osteoarthritis, Japanese White Turmeric
CRP, C-reactive protein; DMARD, Disease Modifying Anti Rheumatic Drugs; GMP, Good Manufacturing Procedure; ITT, Intention To Treat; NSAIDs, Non-Steroidal Anti-Inflammatory Drugs; OA, Osteoarthritis; PGAD, Patients Global Assessment of Disease Severity; PP, Per Protocol; TNF-alpha, Tumour Necrotic Factor-alpha; US FDA, Food and Drug Administration of United States; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index
Osteoarthritis (OA) is a common disease among younger sportsmen, the middle aged and many elderly people of both sexes. It has long been a subject of interest and debate whether treatments that repair destroyed cartilage in osteoarthritis can be found.1 The state-of-the-art treatment for OA still leans heavily on treating/managing its major symptom, pain. The most common painkillers used in OA are paracetamol, NSAIDs and in rare cases, cyclo-oxygenase inhibitors, codeine and synthetic opioids. Unfortunately, most of these painkillers have terrible side effects like liver and kidney damage, gastrointestinal bleeding, as well as stomach and gut erosion.2–7 Therefore, in the development of new anti-OA agents, the goal should be to generate chondro-protective and anti-inflammatory agents that also, do not present with the undesirable side effects. It has earlier been claimed that glucosamine and chondroitin might be such pertinent solutions for OA.8 However, subsequent research indicated that these treatments might not cover what was promised.9 Within the last 15 years, meta-analyses have shown that herbal remedies like ginger, a certain version of rose-hip and different versions of yellow turmeric, all with high levels of curcumin, can lower pain in OA.10–14 Rose-hip was even reported to reduce the consumption of rescue medication and improve cartilage tissue in vitro.15,16 An extraction of a variant of Curcuma longa L, referred to as white turmeric, contrasts with the well-known orange to yellow turmeric by having a very poor curcumin content, but is rich in labdane di-terpene (see HPLC chromatogram Figure 1). The labdane di-terpene is an essential oil component containing 85-90% labdane 8 (17), 12-diene-15, 16-dial as main component. This molecule is found in trace amounts in ginger and yellow turmeric. Compared to the more common yellow turmeric, the white Japanese turmeric contains 80-100 times the concentration of the mentioned di-terpene. The di-terpene has been shown (in vitro) to inhibit the enzyme that causes degradation of hyaluronic acid. Since hyaluronic acid is considered an important fluid that lubricates joints, the prevention of its breakdown is considered important for maintaining good joint health. Moreover, the compound has also been shown to improve the growth of rat cartilage cells17 (Personal communication: Professor Koichiro Komai, Kindai University, Japan). In addition, the labdane di-terpene is claimed to be anti-inflammatory.14,17 The high labdane di-terpene version of white turmeric, which is grown in Japan was recently tested in an open label study including 60 human volunteers, mean age 64.6 +/-8.2 years who had all osteoarthritis of the knee. They were all treated with White Japanese Turmeric for two months and showed a statistically significant drop in pain score, when measured on a 100 mm visual analogue scale after one and two months of treatment (p<0.001). The drop in pain score after 2 month was approximately 50%.17 Such open-label study warrant a blinded follow-up study of longer duration, before any further conclusion can be made, as the placebo-effect may strongly influence the results.

Figure 1 HPLC chromatogram, giving the retention time of curcumin and labdane di-terpenoids in yellow turmeric (green) and White Japanese Turmeric (red).
The present randomized, double-blinded placebo controlled study, therefore, was undertaken to test if the Japanese White Turmeric, high in the level of labdane di-terpene and very poor in curcumin17 can significantly lower pain, improve daily physical activity, as well as reduce the consumption of rescue medication in elderly volunteers suffering from osteoarthritis of the hip and or knee when testing after one, two, three and six month of treatment, respectively. In contrast to the open label study using one 100 mm visual analogue scale for pain, this study used the standard WOMAC questionnaires especially designed for osteoarthritis18 which is based on the mean pain score of 5 different VAS scales for pain as well as a variety of scales for stiffness and daily activity. As this was the first time to test Japanese White Turmeric in a double-blinded, randomized, placebo-controlled set up, it was decided to also investigate the plant medicine for a possible impact on cognitive function, stress, ability to cope socially, as well as effect on sleep quality. We also investigated if the treatment gave rise to any side effects.
Study design and ethics
This investigator-initiated randomized, double-blinded and placebo-controlled clinical study was conducted in accordance with the Helsinki declaration. Three centres: Aarhus, Skanderborg and Copenhagen were involved and the study, including recruitment and follow up, took place from May 2020 – February 2021. Volunteers, who all signed an informed consent form, were recruited from responders to announcements in local newspapers and posters placed at major bus and railway stations, sport facilities, supermarkets and working places. The protocol invited the volunteers to participate for a 6 month clinical study in which Japanese White Turmeric was compared to placebo. The clinical study was approved by the Ethics Committee (ID:H-19040254) (where the full protocol can also be accessed) and the local data supervision system. The study was also reported to the Clinical Trial Gov. (Identifier NCT04500210) and to the data insurance system.
Test medication, dosage and compliance
The test medicine consisted of 12.8 mg of Japanese White Turmeric extract. Extraction was always performed on whole root of Curcuma Longa L variety Yamakawa. Curcuma Longa L is mentioned in http://www.theplantlist.org. and the present variant grows in the district of Yamakawa. The plant is grown on standardized farming ground and processed in a controlled drying environment. Extraction is performed at a GMP certified factory. Before gelatine encapsulation, which takes place in a GMP factory approved by the Japanese and US FDA, the product is sent to Japan Food Research Laboratory for qualitative and quantitative analysis of the active, labdane diterpene. A minimum of at least 0.22 mg/capsule of this diterpene is always guaranteed. This dosing is a little higher than what was used in the open label study17 as the weight of the population in Europe is a little higher than what is observed in Japan.
The placebo was a similar sized gelatine capsule of similar colour, taste and odour. Batch numbers for active and placebo was R9G23F and R9G22F, respectively. Computer guided randomization (1:1) of the study participants was performed in blocks of 10. The study code was kept by Veritas Ltd, Tokyo, who also took care of the production and sequential labelling of study medication. Thus, the volunteers were randomly allocated to active treatment (n=60) or placebo (n=60) groups, each administered one daily gelatine capsule, to be taken in the morning together with the breakfast. And the purpose was to test the effect of the labdane di-terpene on symptoms of osteoarthritis and to look for possible side effects. The CONSORT Harms 2022 statement was considered. All volunteers were invited to call the study nurses in case of any suspicion of a side effect at any time of the day. Furthermore, on each telephone contact to the volunteers they were, at the end of the conversation, asked about any possible side effect had occurred. And on the visiting to the clinic after 3 and 6 month the volunteers furthermore had the possibility to report side effects and they were also, always at the end of the visit asked directly about possible side effects.
Compliance was simply calculated by counting the capsules returned by the volunteers after 3 and 6 months of treatment, respectively. Compliance was finally given as percentages.
Participants
A total of 120 volunteers, represented by both sexes, aged 40 years and above, with mild to moderate osteoarthritis (OA) of the hip and/or knee, were included in this double-blinded, randomized, placebo controlled clinical trial. Allocation to either active treatment or placebo was carried out in blocks of 10 by a computer program, and the volunteers as well as the entire staff around the participants, including doctors and nurses were all blinded until the code was broken after the study had been finished. Veritas Ltd generated the random allocation sequence, doctors enrolled the volunteers and nurses assigned participants to intervention. During the study period assessors as well as care providers were only aware the number on the bottle given to that particular volunteer they were assessing/supporting. The trial ended when the last included volunteer ended his/her 6 month treatment period. During the entire study period, a volunteer would always meet the same staff and be interviewed and tested in the same room.
Inclusion criteria: To be included in the study volunteers should be a male or a female of more than 40 years of age. There should be symptoms of hip and/or knee osteoarthritis for more than 6 month and a reported pain score of 3 or more on a numerical scale from 0-10 (10 worst possible). Morning stiffness should also be reported. And OA should be diagnosed according to the criteria of the American College of Rheumatology.19,20 Volunteers on rescue medication like paracetamol, NSAID´s, codeine and tramadol could be included in the study, but were asked to register their daily consumption of such medication in a diary during the whole entire study.
Exclusion criteria: Volunteers who had been treated with glucosamine, chondroitin sulphate, intra articular hyaluronate, systemic or intra-articular glucocorticoids, TNF-alpha inhibitors, DMARD, herbal remedies with documented impact on symptoms from OA like - ginger, rose-hip, avocado-soyabean etc., within the last 3 month before the screening period were not included. Moreover patients, if on any rescue medication, should maintain a stable drug dose during the two weeks prior to screening. A change in physical activity (upstarts of training programs) or change in strategy for diet, during the study period, were causes for exclusion. Volunteers suffering from joint disease other than OA, abuse of alcohol and drugs, psychiatric diseases, known allergies, fibromyalgia, substantial abnormalities in haematological parameters, hepatic, renal or other metabolic dysfunctions, or who were waiting for planned major surgery, or had participated in other clinical trial within the last 3 months, or had difficulties with adhering to the study protocol, were also excluded.
Outcome measures
Primary outcomes: WOMAC pain and WOMAC activity of daily living (ADL) tested after the third and sixth month of the study period.
Secondary outcomes: WOMAC stiffness and patients’ global assessment of disease severity (PGAD) tested after 3 and 6 months of treatment. Other secondary end points criteria included overall impact of treatment on pain on a scale from 0 (no impact at all) to 4 (very satisfying) tested after 3 and 6 months, respectively; Cognitive function tested as Mini Mental State Examination (MMSE); Memory and Concentration (Wechsler), sleeping quality (100 VAS scale), safety of the two treatments, by simply counting the numbers of side effects reported and level of biochemical markers such as CRP, total Leucocytes and Cholesterol. The latter were tested initially for basal levels and again after the 3- and 6-months check-up.
Additional parameters including what was self-recorded in patients’ diaries: Pain in hip and or knee evaluated as the worst possible experienced during the previous 7 days was recorded on a numerical scale from 0-10, initially and after 3 and 6 months, respectively. Consumption of rescue medication such as Paracetamol, NSAIDs, and synthetic opioids were daily recorded in a diary. The data given on such rescue medication is calculated as the mean of the first 14 days of treatment for each volunteer vs the mean of the last 14 days of the 3 months treatment period and the 6 months treatment period, respectively. During data analyses (see below), the numbers of volunteers in the per protocol (PP) analysis group who were on rescue medication were also recorded initially and after 6 months, in the treatment and placebo groups, for estimation of how many volunteers were simply able to quit taking NSAIDs and paracetamol during the study. The study participants were also instructed to make daily records of their evaluations of mood, energy, wellbeing and sleeping quality in the diaries provided. This was done on a numerical scale from 0 to 10 (10 worst possible).
Power estimation and statistical evaluation
It was planned to include 60 volunteers in each of the two arms, to assure that at least 50 volunteers in each arm would finish the trial. Data from a trial of similar type indicated that this number of volunteers would be relevant for a pilot study.16 With the present number of volunteers, the risk of a type 1 error was calculated to be less than 5% and the risk of a type error less than 20%. The two groups were also balanced with respect to the different medical ailments they were suffering from and as regards to the treatment they were placed on by their respective general practitioners. When “effect size” is mentioned, it is calculated as the impact form active treatment minus the impact from placebo.
Statistical evaluation: Data are given as mean values ± SD (except for effect size, expressed as values ± SEM) when comparing within groups and when comparing active and placebo treatments. Data are evaluated on the bases as per protocol (PP) for patients who participated for the entire 6-month study period, as well as, based on intention to treat (ITT), whereby the last reported values are carried forward for missing data. The Wilcoxon test was used for within group comparisons. Friedman´s test was used to evaluate changes within groups over time while the Mann-Whitney test was applied for comparing groups. Effect size, when given, is the difference between placebo score and active treatment evaluated at the same timepoint.
Patients’ characterization
Two hundred and thirty-two individuals responded to our advertisement. Of these, 129 did not satisfy the inclusion criteria. Seven of these responders, we recognized, would have difficulties to adhere to the protocol, 9 had in addition another joint disease than OA, 10 suffered fibromyalgia, 12 had either problems with alcohol or drugs, 13 had taken some of the forbidden herbal remedies within the last 3 month, 31 did not score enough on the scale for pain and the remaining 47 were on treatment or had recently been treated glucocorticoids, DMARD, hyaluronic acid or glucosamine. This left 120 volunteers, of which 60 were given placebo and 60 were actively treated, for inclusion in the study. Of these 78 were women and 42 were men. Mean age was 68 years, with a range 43-86. The volunteers had suffered osteoarthritis for a mean period of 8.5 years, range 1-33 years. Specifically, 67 volunteers presented with osteoarthritis of the knee, 17 had osteoarthritis of the hip and 36 had osteoarthritis of both the hip and knee. Further details on the two groups of the included volunteers are given in table 1 demographics.
|
|
Active |
Placebo |
|
||
|
Item |
Mean |
SD |
Mean |
SD |
P-value |
|
Number of volunteers (men, women) |
59 (18,41) |
|
50 (20,30) |
|
0.614 |
|
Age |
67.71 |
08.66 |
68.86 |
08.52 |
0.499 |
|
Body weight |
77.13 |
15.59 |
81.67 |
17.89 |
0.271 |
|
BMI |
25.98 |
04.20 |
27.51 |
05.53 |
0.316 |
|
Pamol mg (total amount taken by group) |
4.156 |
8.947 |
5.576 |
11.095 |
0.825 |
|
NSAIDs mg (total amount taken by group) |
355.9 |
860 |
568 |
2.646 |
0.062 |
|
Number of patients on paracetamol |
25 |
|
21 |
|
0.900 |
|
Number of patients on NSAIDs |
13 |
|
4 |
|
0.250 |
|
Pain on 10 step numerical scale |
05.32 |
1.45 |
5.41 |
1.70 |
0.673 |
|
WOMAC pain |
18.85 |
8.88 |
17.67 |
8.33 |
0.477 |
|
WOMAC activity of daily living (ADL) |
60.89 |
31.26 |
56.69 |
31.29 |
0.446 |
|
Number of patients with OA of the knee |
9 |
|
7 |
|
0.950 |
|
Number op patients with OA of the hip |
31 |
|
29 |
|
0.500 |
|
Number of patients with OA of hip/knee |
19 |
|
14 |
|
0.550 |
|
General wellbeing |
7.00 |
1.68 |
6.88 |
1.60 |
0.677 |
|
Sleeping quality |
6.75 |
1.87 |
6.91 |
1.66 |
0.720 |
Table 1 Demographics for the Placebo and Active treatment groups
*OA, Osteoarthritis.
At the start of the experiment, 11 volunteers (1 from the active treatment group and 10 on placebo), had to be excluded from the study, due to protocol violation. The reason for protocol violation was that these study participants started up a personal physical training program (GLAD), created and newly lanced by “The Danish Society against Arthritis”. Thus, there were now 59 volunteers in the actively treated group and 50 on placebo (for details see Study Flow-Chart, Figure 2).
During the first month, 8 volunteers, seven of which were on active treatment left the study. In the active treatment group, one person simply did not turn up and we could not get in contact with him. Later his general practitioner told us that he just had changed his mind regarding signing up. Another patient died from a heart attack (this could not be associated to treatment) and his wife, who also participated in the study decided to withdraw. The remaining four volunteers and the one who withdrew from the placebo group, left for personal reasons. Consequently, a total of 52 on active treatment and 49 on placebo went on for the next 2 months treatment period. After finishing the three month test, twelve on active treatment and twenty two on placebo treatment decided not to continue and so left the study. The reasons for leaving the study were as follows: a) the medication did not work (most volunteers leaving after three months (n=22) were in fact treated placebo) and b) many of the volunteers found it very time-consuming filling in diaries on symptom scores and to register the use of rescue medication. As none of the volunteers, who decided to continue, left the study during the last 3 month we ended up with a per protocol population of 67 volunteers after 6 months. Forty of these were on turmeric extract and 27 were on placebo. Details in flow chart.
Primary endpoints
WOMAC Pain: The per protocol (PP) scores for WOMAC pain significantly declined from the first month to the 3rd month irrespective of treatment. For details see Figure 3 and Table 2. After three month of treatment, there were no significant difference between the two treatments although there was a trend in favour of active treatment. Effect size 0.846 ± 1.844, n=52 for treatment versus n=49 for placebo (95% confidence interval -4.504 to 2.812) (p<0.647). However, after 6 months treatment, the pain score observed as the result of active treatment remained significantly lower than the initial score and the 6 month placebo score have more or less returned to the initial level for the placebo group resulting in a Man-Whitney (p< 0.041). Effect size 4.961 ± 2.366, n=40 for treatment versus n=27 for placebo (95% confidence interval -9.686 to -0.2359) (p< 0.039). The drop in pain score in the actively treated group was close to 30% after 6 month as compared to a modest 2% drop observed for placebo. Details in Figure 3 and Table 2. The reduction in pain during the first three month of placebo treatment, however, is not so surprising as a meta-analysis indicate that more than 50 percentage of osteoarthritis patients reports less pain, when treated placebo.21 When Friedmans test was applied on ITT as well as on PP data, there was a significant improvement in pain score after 3 and after 6 month, irrespective of treatment: active treatment p<0.000 and placebo p<0.017, respectively.
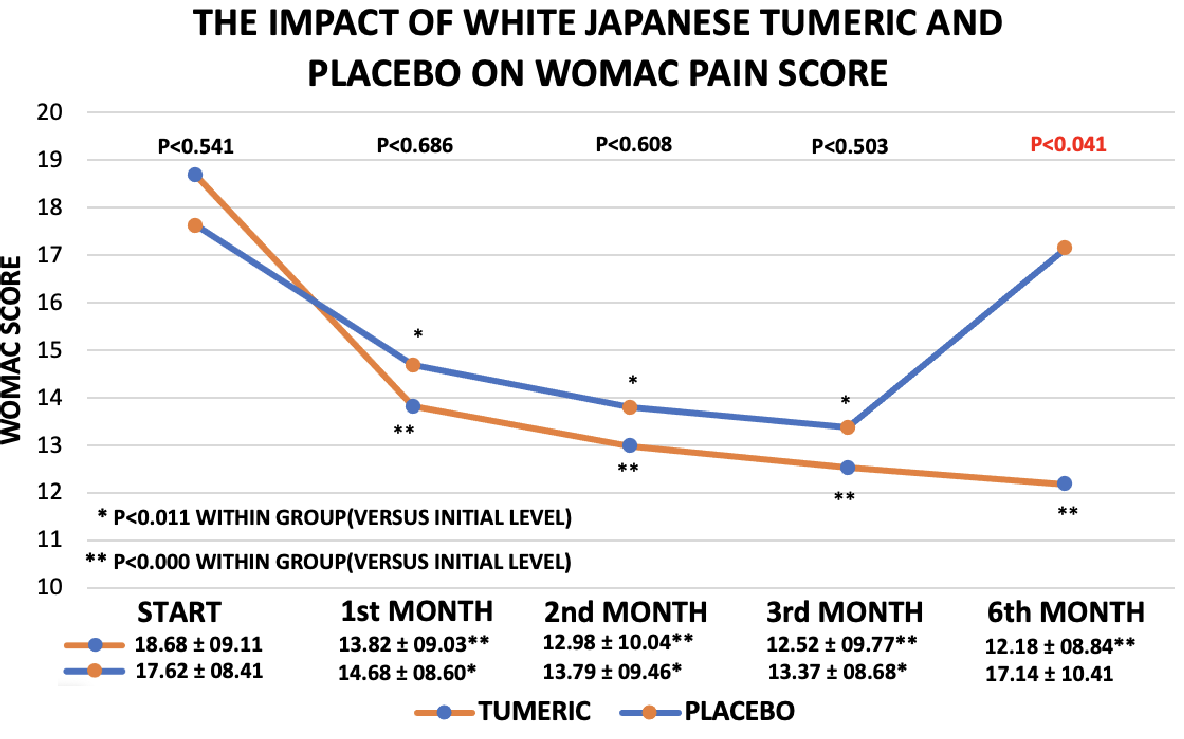
Figure 3 WOMAC Pain scores for the Placebo and active treatments during the study (per protocol analysis). At the bottom, a table is inserted, providing the mean +/- SD is for each time point of the two treatments.
|
|
|
Active |
Placebo |
|
||||||
|
Item |
Month |
N |
Mean |
SD |
Wilcoxon |
N |
Mean |
SD |
Wilcoxon |
Mann-Whitney |
|
Pain |
Baseline |
59 |
18.85 |
08.88 |
|
50 |
17.67 |
08.33 |
|
0.4774 |
|
|
Month 1 |
59 |
14.57 |
09.03 |
|
50 |
14.78 |
08.54 |
|
0.9988 |
|
|
DIFF of 1 to base |
59 |
04.28 |
08.46 |
0.0006 |
50 |
02.88 |
07.92 |
0.0109 |
0.5495 |
|
|
Month 2 |
59 |
13.83 |
09.98 |
|
50 |
13.91 |
09.40 |
|
0.9336 |
|
|
DIFF of 2 to base |
59 |
05.03 |
09.25 |
0.0002 |
50 |
03.76 |
07.88 |
0.0028 |
0.5235 |
|
|
Month 3 |
59 |
13.42 |
09.79 |
|
50 |
13.50 |
08.64 |
|
0.8809 |
|
|
DIFF of 3 to base |
59 |
05.43 |
08.81 |
<0.0001 |
50 |
04.17 |
07.95 |
0.0005 |
0.4209 |
|
|
Month 6 |
59 |
14.24 |
09.51 |
|
50 |
15.89 |
10.22 |
|
0.4281 |
|
|
DIFF of 6 to base |
59 |
04.61 |
07.86 |
<0.0001 |
50 |
01.78 |
09.05 |
0.0545 |
0.1276 |
|
|
DIFF months 3/6 |
59 |
-00.88 |
05.34 |
0.2905 |
50 |
-2.40 |
06.75 |
0.0203 |
0.3145 |
|
Physical Activity |
Baseline |
59 |
60.89 |
31.26 |
|
50 |
56.69 |
31.29 |
|
0.4460 |
|
|
Month 1 |
59 |
48.46 |
30.96 |
|
50 |
49.20 |
29.08 |
|
0.8380 |
|
|
DIFF of 1 to base |
59 |
12.43 |
27.16 |
0.002 |
50 |
07.49 |
28.18 |
0.1214 |
0.3480 |
|
|
Month 2 |
59 |
45.21 |
33.72 |
|
50 |
45.82 |
33.69 |
|
0.8904 |
|
|
DIFF of 2 to base |
59 |
15.68 |
28.93 |
<0.0001 |
50 |
10.87 |
33.67 |
0.0716 |
0.1956 |
|
|
Month 3 |
59 |
45.64 |
31.07 |
|
50 |
48.18 |
30.35 |
|
0.6333 |
|
|
DIFF of 3 to base |
59 |
15.26 |
25.53 |
<0.0001 |
50 |
08.50 |
26.59 |
0.0810 |
0.0924 |
|
|
Month 6 |
59 |
45.55 |
31.80 |
|
50 |
52.10 |
29.43 |
|
0.2314 |
|
|
DIFF of 6 to base |
59 |
15.35 |
25.21 |
<0.0001 |
50 |
04.59 |
25.68 |
0.2326 |
0.0296 |
|
|
DIFF months 3/6 |
59 |
00.09 |
14.03 |
0.5545 |
50 |
-03.91 |
12.89 |
0.1153 |
0.1328 |
Table 2 ITT data analysis of WOMAC pain and WOMAC activity of daily living (ADL)
WOMAC ADL: Activity scores significantly declined as the result of active treatment, which indicates improved physical activity in daily life after already 1, 2 and 3 month of treatment. Effect size after 3 month was 6.680 ± 5.974 (95% confidence interval -18.53 to 5.173) (p<0.266). In accordance with the pain scores the most pronounced impact on daily activity (ADL) occurred after 6 months. In addition, after the 6 months’ time span there is a significant difference between active treatment and placebo (p<0.028)(Figure 4). And effect size was now 12.87 ± 6.908 (95% confidence interval -26.67 to 0.9257) (p< 0.067). When focusing on within group analysis, placebo treatment did not, at any time, result in any statistically significant improvements, although there was a trend to some improvement, which reached its optimum after 2 months of treatment (Figure 4). The changes in activity scores with placebo is very much in contrast to the active treatment with Turmeric, where a highly significant improvement is obtained already after 1 month of treatment (p<0.001). The improvement is maintained during the entire 6 months treatment period. Specifically, the improvement in physical activity (29%) after 3 month and (38%) after 6 months as compared to a modest and insignificant (6%) improvement in the placebo group, p<0.028, comparing groups after 6 month, should be noted. Friedman´s test whether applied on ITT or PP data was highly statistically significant after 3 month of treatment and after 6 month when testing actively treated volunteers, p<0.000 and p<0.000, respectively. This was in contrast to placebo 3 and 6 month values, yielding p values of p<0.150 and p<0.306, respectively.
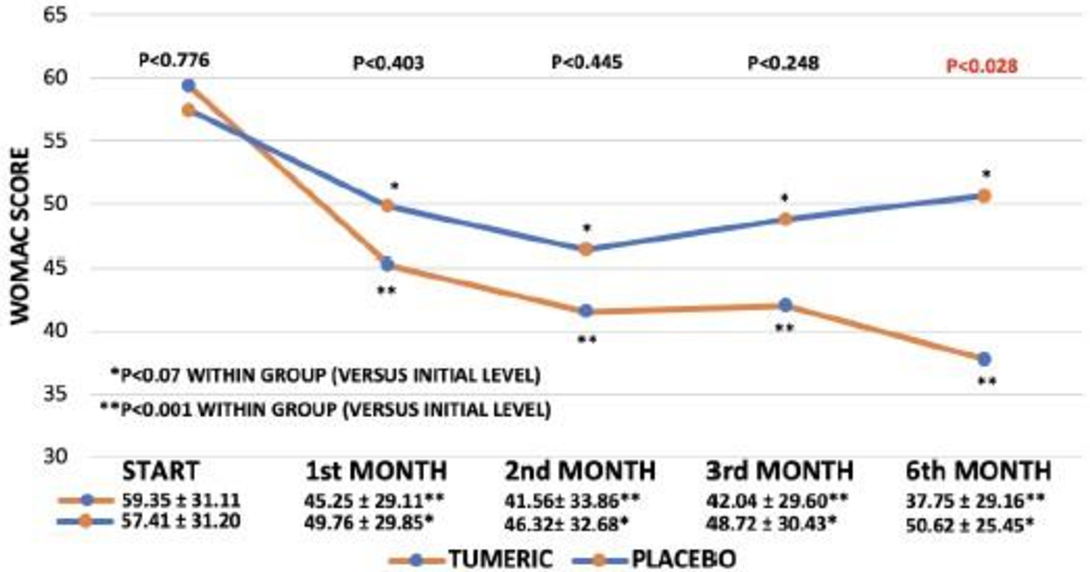
Figure 4 WOMAC Activity of Daily Living (ADL) scores for the Placebo and Turmeric treatments during the study (per protocol analysis). At the bottom, a table is inserted to show the mean +/- SD for each time point in the two treatments.
Secondary endpoints
WOMAC stiffness: The baseline data of the two experimental groups are not identical as regards the level of stiffness of limbs. As shown in Table 3a, the actively treated group claimed to have more stiffness than did the placebo group. The results on stiffness should therefore be taken with some precaution. However, the same pattern of improvement with active treatment for pain and ability to perform daily activity is observed when considering effect of Turmeric on stiffness. After one month of treatment, there is a significant decline in stiffness in the Turmeric group (p<0.0001) whereas no significant reduction is observed in the placebo group. Both active and placebo treatments resulted in significant reductions in stiffness after 2 and 3 months. However, after 3 months of treatment, the drop in the actively treated group is superior to the drop observed in placebo (p<0.021), table 3a. After 6 months of treatment, the drop in stiffness is still highly significant (p<0.000) in the Turmeric group, whereas the impact from placebo has vanished at that time point. The delta drop in stiffness in the active group in the final stage of the study is 4.59 +/-4.41 as compared to - 0.28 +/-5.05 in the placebo group (p<0.0001) comparing groups (Table 3a). ITT analysis (Table 3b) support the PP data indicating a statistically significance between-group-differences in delta drop after 6 months treatment of P<0.003, in favour of Turmeric. Friedman´s test did not distinguish between the two groups, p< 0.002, irrespective of treatment when testing after 3 and 6 month.
|
|
|
ACTIVE |
PLACEBO |
|
||||||
|
Item |
Month |
N |
Mean |
SD |
#Wilcoxon |
N |
Mean |
SD |
Wilcoxon |
Mann-Whitney |
|
Stiffness |
Baseline |
59 |
10.46 |
05.09 |
|
50 |
08.44 |
04.22 |
|
0.0343 |
|
|
Month 1 |
52 |
07.84 |
04.64 |
|
49 |
07.47 |
04.93 |
|
0.5390 |
|
|
DIFF of 1 to base |
52 |
02.99 |
04.49 |
<0.0001 |
49 |
01.05 |
04.38 |
0.1282 |
0.0416 |
|
|
Month 2 |
52 |
06.28 |
04.80 |
|
49 |
07.17 |
04.93 |
|
0.3594 |
|
|
DIFF of 2 to base |
52 |
04.55 |
05.35 |
<0.0001 |
49 |
01.35 |
04.69 |
0.0272 |
0.0019 |
|
|
Month 3 |
52 |
06.73 |
05.03 |
|
49 |
06.55 |
04.19 |
|
0.9474 |
|
|
DIFF of 3 to base |
52 |
04.09 |
05.47 |
<0.0001 |
49 |
01.97 |
03.93 |
0.0001 |
0.0215 |
|
|
Month 6 |
40 |
06.09 |
04.47 |
|
27 |
08.40 |
04.81 |
|
0.0527 |
|
|
DIFF of 6 to base |
40 |
04.59 |
04.41 |
<0.0001 |
27 |
-0.28 |
05.05 |
0.5659 |
<0.0001 |
|
|
DIFF months 3/6 |
40 |
-0.34 |
03.64 |
0.6923 |
27 |
-2.39 |
03.91 |
0.0025 |
0.0194 |
|
HTW |
Month 1 |
52 |
00.71 |
01.13 |
|
49 |
00.63 |
00.99 |
|
0.8592 |
|
|
Month 2 |
52 |
01.10 |
01.35 |
|
49 |
00.53 |
00.98 |
|
0.0318 |
|
|
DIFF to month 1 |
52 |
-0.39 |
01.12 |
0.0033 |
49 |
00.10 |
00.85 |
0.6658 |
0.0228 |
|
|
Month 3 |
52 |
01.31 |
01.44 |
|
49 |
00.81 |
01.14 |
|
0.0917 |
|
|
DIFF to month 1 |
52 |
-0.60 |
01.36 |
0.0006 |
49 |
-0.17 |
00.86 |
0.0926 |
0.0225 |
|
|
Month 6 |
40 |
02.19 |
01.42 |
|
27 |
01.69 |
01.87 |
|
0.0801 |
|
|
DIFF to month 1 |
40 |
-1.32 |
01.73 |
<0.0001 |
27 |
-0.91 |
01.99 |
0.0347 |
0.0858 |
|
|
DIFF months 3/6 |
40 |
-0.72 |
01.73 |
0.0157 |
27 |
-0.56 |
01.97 |
0.1775 |
0.6610 |
|
PGADS |
Baseline |
59 |
05.28 |
02.29 |
|
50 |
04.48 |
02.10 |
|
0.0744 |
|
|
Month 1 |
52 |
03.63 |
02.14 |
|
49 |
04.26 |
02.17 |
|
0.1179 |
|
|
DIFF of 1 to base |
52 |
01.51 |
02.28 |
<0.0001 |
49 |
00.26 |
02.17 |
0.7579 |
0.0013 |
|
|
Month 2 |
52 |
03.26 |
02.32 |
|
49 |
03.64 |
02.47 |
|
0.4948 |
|
|
DIFF of 2 to base |
52 |
01.89 |
02.47 |
<0.0001 |
49 |
00.89 |
02.09 |
0.0117 |
0.0162 |
|
|
Month 3 |
52 |
03.63 |
02.13 |
|
49 |
04.12 |
02.10 |
|
0.3201 |
|
|
DIFF of 3 to base |
52 |
01.52 |
02.44 |
<0.0001 |
49 |
00.40 |
01.74 |
0.1001 |
0.0064 |
|
|
Month 6 |
40 |
02.75 |
01.99 |
|
27 |
03.82 |
02.70 |
|
0.1430 |
|
|
DIFF of 6 to base |
40 |
02.56 |
02.59 |
<0.0001 |
27 |
00.34 |
02.44 |
0.3459 |
0.0010 |
|
|
DIFF months 3/6 |
40 |
00.59 |
02.47 |
0.4182 |
27 |
-0.304 |
02.52 |
0.6066 |
0.3482 |
Table 3a Per protocol analysis data of WOMAC stiffness, how treatment did work (HTW), given on a scale from 0 (no impact) to 4 (excellent). Data were available from the end of first month and throughout. Patients’ global evaluation of disease severity (PGAD) is also presented
|
|
|
Active |
Placebo |
|
||||||
|
Item |
Month |
N |
Mean |
SD |
Wilcoxon |
N |
Mean |
SD |
Wilcoxon |
Mann-Whitney |
|
Stiffness |
Baseline |
59 |
10.46 |
05.09 |
|
50 |
08.44 |
04.22 |
|
0.0343 |
|
|
Month 1 |
59 |
07.83 |
04.63 |
|
50 |
07.42 |
04.89 |
|
0.5021 |
|
|
DIFF of 1 to base |
59 |
02.63 |
04.32 |
<0.0001 |
50 |
01.02 |
04.34 |
0.1323 |
0.0779 |
|
|
Month 2 |
59 |
06.45 |
04.79 |
|
50 |
07.12 |
04.89 |
|
0.4791 |
|
|
DIFF of 2 to base |
59 |
04.01 |
05.23 |
<0.0001 |
50 |
01.32 |
04.64 |
0.0256 |
0.0127 |
|
|
Month 3 |
59 |
06.85 |
04.98 |
|
50 |
06.51 |
04.16 |
|
0.9457 |
|
|
DIFF of 3 to base |
59 |
03.61 |
05.30 |
<0.0001 |
50 |
01.93 |
03.90 |
0.0001 |
0.1075 |
|
|
Month 6 |
59 |
07.08 |
04.85 |
|
50 |
07.80 |
04.52 |
|
0.4054 |
|
|
DIFF of 6 to base |
59 |
03.38 |
04.84 |
<0.0001 |
50 |
00.61 |
04.29 |
0.2520 |
0.0030 |
|
|
DIFF months 3/6 |
59 |
-00.23 |
02.99 |
0.7641 |
50 |
-01.29 |
03.09 |
0.0022 |
0.0493 |
|
HELMED |
Month 1 |
59 |
00.63 |
01.08 |
|
50 |
00.62 |
00.99 |
|
0.8638 |
|
|
Month 2 |
59 |
00.97 |
01.31 |
|
50 |
00.52 |
00.97 |
|
0.0864 |
|
|
DIFF to month 1 |
59 |
-00.34 |
01.06 |
0.0067 |
50 |
00.10 |
00.84 |
0.6658 |
0.0290 |
|
|
Month 3 |
59 |
01.15 |
01.41 |
|
50 |
00.79 |
01.13 |
|
0.2419 |
|
|
DIFF to month 1 |
59 |
-00.53 |
01.29 |
0.0005 |
50 |
-00.17 |
00.85 |
0.1851 |
0.0480 |
|
|
Month 6 |
59 |
01.64 |
01.52 |
|
50 |
01.09 |
01.58 |
|
0.0311 |
|
|
DIFF to month 1 |
59 |
-01.01 |
01.31 |
<0.0001 |
50 |
-00.47 |
01.62 |
0.0866 |
0.0101 |
|
|
DIFF months 3/6 |
59 |
-00.48 |
01.46 |
0.0379 |
50 |
-00.30 |
01.46 |
0.1775 |
0.5200 |
|
PGADS |
Baseline |
59 |
05.28 |
02.29 |
|
50 |
04.48 |
02.10 |
|
0.0744 |
|
|
Month 1 |
59 |
03.95 |
02.24 |
|
50 |
04.22 |
02.16 |
|
0.4623 |
|
|
DIFF of 1 to base |
59 |
01.33 |
02.20 |
<0.0001 |
50 |
00.26 |
02.15 |
0.7685 |
0.0029 |
|
|
Month 2 |
59 |
03.62 |
02.44 |
|
50 |
03.61 |
02.45 |
|
0.9336 |
|
|
DIFF of 2 to base |
59 |
01.66 |
02.40 |
<0.0001 |
50 |
00.87 |
02.08 |
0.0128 |
0.0409 |
|
|
Month 3 |
59 |
03.94 |
02.24 |
|
50 |
04.09 |
02.09 |
|
0.8522 |
|
|
DIFF of 3 to base |
59 |
01.34 |
02.34 |
<0.0001 |
50 |
00.39 |
01.73 |
0.2033 |
0.0166 |
|
|
Month 6 |
59 |
03.54 |
02.35 |
|
50 |
04.25 |
02.56 |
|
0.1548 |
|
|
DIFF of 6 to base |
59 |
01.74 |
02.73 |
<0.0001 |
50 |
00.23 |
02.22 |
0.8053 |
0.0011 |
|
|
DIFF months 3/6 |
59 |
00.40 |
02.04 |
0.4509 |
50 |
-00.16 |
01.84 |
0.7376 |
0.4199 |
Table 3b ITT data analysis of WOMAC stiffness, how treatment did work (HTW), given on a scale from 0 (no impact) to 4 (excellent). Data were available form the end of the first month and throughout. Patients’ global evaluation of disease severity (PGAD) is also given
Patients’ global assessment of disease severity (PGAD)
In line with the effects on the OA symptoms, there is a highly significant (p<0.0001) improvement in the actively treated group when comparing the patients’ assessment of the severity of their illness at the different treatment periods with baseline (Table 3a). In the placebo group there is only a modest significant improvement in within group differences after 2 months (p<0.011). When comparing groups, there is a statistically significant difference between the delta changes registered with active treatment, as compared to the modest delta changes recorded month by month with placebo (Table 3a). This is evident after 1, 2, 3 and 6 months respectively, p<0.001, p<0.016, p<0.006 and p<0.001, respectively. Similar levels of significance are observed when evaluating the delta PGAD values of both groups by ITT analysis (Table 3b). Friedman´s test was significant at the p<0.000 level for active treatment after 3 and also after 6 month of treatment, whereas placebo did not attain statistical significance at the 3 and 6 month level, p<0.0515 and p<0.0675, respectively. Similar results for ITT and PP values.
Quality of treatment given by patients on a scale from 0 (no impact at all) to 4 (excellent)
After 1, 2, 3 and 6 month of treatment, the volunteers were asked to assess how they felt the treatment has worked for them (HTW). The pattern was identical to previous analysis of OA symptoms and PGAD. PP data indicated that the actively treated group significantly felt improvements (i.e., the volunteers felt that the treatment worked) from the second month of treatment (p<0.003) and onwards. By contrast, a modest statistically significant improvement was only indicated by the placebo group after 6 months (p<0.037). Mann–Whitney analytic comparison of the groups also yielded levels of statistical significance in favour of active treatment from month 2 and onwards and the level of significance was even more pronounced in the ITT analysis (Tables 3a and 3b). For active treatment, Friedman´s test resulted in highly significant p values after 3 and 6 month, p<0.000 and p<0.000 respectively. Placebo did not attain significance after 3 month (p<0.084 but was significant after 6 month p<0.025. ITT and PP values yielded similar results.
Cognitive Functions (memory/concentration) and social ability and Mini Mental State Examination (MMSE) were administered initially and after 3 month of the study and did not show any impact from either treatment. There were no significant differences within and between groups when comparing data from Wechlers memory scale. And stress and ability to cope socially after the third and sixth month was not changed by any of the treatments. (Data not shown).
Sleeping quality (data only available after 3 months of the study). There was a significant improvement in sleeping quality within the actively treated group (p<0.028). Such improvements were not observed in the placebo group. However, there was no significant differences comparing groups (p<0.404). (Data not shown).
Safety
The treatments were well tolerated by both groups. No serious side effects were reported. A mild side effect in the form of skin rashes, was reported in 3 volunteers from each of the placebo and active treatment groups. The two groups represented equal numbers of episodes with fewer, cold, flue, cough and headaches.
Biochemistry
All questionnaires were mandatory to volunteers. However, blood sampling was not mandatory. For that reason, blood was only sampled from 47 of the actively included and from 44 in the placebo group. There was no statistically significant alterations in total cholesterol, within groups or between groups. Total leucocytes count tended to decline more in the actively treated group than in the placebo (p<0.066). There were no statistically significant differences in C-reactive protein (CRP) when comparing groups. (Data not given).
Weight, heart rate and blood pressure: After 3 and 6 months, there was no significant change within groups or between groups (p<0.621) when body weight was evaluated. Blood pressures did not differ comparing groups after 3 and 6 months, p<0.363 and p<0.331), respectively. Heart rate was not influenced by either treatment. (Data not given).
Compliance
Compliance in the actively treated group was 96.06 +/- 05.51 percentage after 3 month and 93.10 +/- 13.46 percentage after 6 months. By comparison in the placebo group, compliance was 96.87 +/- 4.32 percentage and 97.45 +/- 14.63 percentage after three and six months, respectively. There were no statistically significant difference comparing groups at the three- and six-month level.
Other parameters tested
Worst experienced pain during the last 7 days: Scored on a numerical 0-10 scale upon inclusion and after 3 and 6 months: The within group and between group evaluations are presented in Table 4a (PP evaluation) and in Table 4b (ITT evaluation). Irrespective of treatment, there was a significant reduction in pain score after 3 and 6 months respectively, when evaluating within the groups. However, when evaluating between groups, Japanese turmeric was strongly superior to placebo after 3 month (p<0.017) and also after 6 month (p<0.002) of treatment. It was also shown that the 6 month delta score was significantly improved when compared to the three month level (p<0.039) suggesting benefit of longer treatment. Details in Table 4a and b indicating that per protocol and intention to treat analyses yielded similar results.
|
Item |
Month |
Active |
Placebo |
|
Mann-Whitney |
|||||
|
|
|
N |
Mean |
SD |
Wilcoxon |
N |
Mean |
SD |
Wilcoxon |
|
|
Incl VAS |
Baseline |
59 |
05.32 |
01.36 |
|
50 |
05.41 |
01.69 |
|
0.6708 |
|
|
3 |
52 |
03.75 |
01.86 |
|
49 |
04.53 |
02.06 |
|
0.0744 |
|
|
DIFF of 3 mo. to base |
52 |
01.57 |
01.90 |
<0.0001 |
49 |
00.88 |
01.70 |
<0.0001 |
0.0179 |
|
|
6 |
40 |
03.18 |
01.89 |
|
27 |
05.02 |
02.39 |
|
0.0012 |
|
|
DIFF of 6 mo. to base |
40 |
01.98 |
01.86 |
<0.0001 |
27 |
00.35 |
02.16 |
0.3430 |
0.0022 |
|
|
DIFF of 3 mo. to 6 mo |
40 |
00.15 |
01.41 |
0.3849 |
27 |
-0.80 |
02.26 |
0.0863 |
0.0399 |
Table 4a Per protocol analysis of effects of treatments on pain score, evaluated at the inclusion and after 3 and 6 months of treatment, respectively. The score is given as the worst experienced pain in hip and/or knee join during the last 7 days on a numerical scale from 0 (no pain) – 10 (much pain)
|
Item |
Month |
Active |
Placebo |
|
Mann-Whitney |
|||||
|
|
|
N |
Men |
SD |
Wilcoxon |
N |
Mean |
SD |
Wilcoxon |
|
|
Incl VAS |
Baseline |
59 |
05.32 |
01.36 |
|
50 |
05.41 |
01.69 |
|
0.6708 |
|
|
3 |
59 |
03.76 |
01.75 |
|
50 |
04.53 |
02.04 |
|
0.0468 |
|
|
DIFF of 3 mo. to base |
59 |
01.56 |
01.78 |
<0.0001 |
50 |
00.88 |
01.70 |
<0.0001 |
0.0134 |
|
|
6 |
59 |
03.63 |
01.96 |
|
50 |
04.97 |
02.13 |
|
0.0015 |
|
|
DIFF of 6 mo. to base |
59 |
01.69 |
01.93 |
<0.0001 |
50 |
00.44 |
01.71 |
0.0284 |
0.0002 |
|
|
DIFF of 3 mo. to 6 mo. |
59 |
00.13 |
01.16 |
0.0787 |
50 |
-0.44 |
01.70 |
0.1009 |
0.0088 |
Table 4b ITT analysis of effect of treatments on pain score, evaluated at the inclusion and after 3 and 6 months of treatment, respectively. The score is given as the worst experienced pain in hip and/or knee joint during the last 7 days on a numerical scale form 0 (no pain) to 10 (much pain)
Diaries for paracetamol and NSAIDs
The intake of rescue medication was limited to paracetamol and NSAIDs, except in one person from the active treatment group, and two in the placebo group who had been prescribed the synthetic opioid, tramadol by their physician (for details see demographics in Table 1). At the end of the study tramadol was not taken by any of the volunteers. As the number of volunteers on opioids was so small, we only report data on paracetamol and NSAIDs.
The initial amount of paracetamol intake, as reported in the patients’ diaries of the actively treated group, was 4.156 +/- 8.998 mg which significantly dropped to 1.996 +/- 5.254 mg (p<0.008) after three month of treatment. This should be compared to a modest increase in the consumption of paracetamol in the placebo group: Initial level 5.576 +/- 11.095 mg versus the 3-months level of 5.648 +/- 10.720 mg (p<0.419). (Details in Figure 5). When comparing groups at the 3-months level, there was a drop in consumption of paracetamol of 52% (p<0.014) in favour of active treatment. The data for pain-medication at 6 months’ treatment is less solid, as these are not extracted from diaries, but based on directly asking the volunteers during their 6th month clinic visit. However, the intake level of paracetamol in the actively treated group, after 6 month was reported as 1.138 +/- 4.496 mg – compared to 4.320 +/-12.001 mg in the placebo group, a drop of 72% p<0.050, when comparing the two groups (Figure 5).
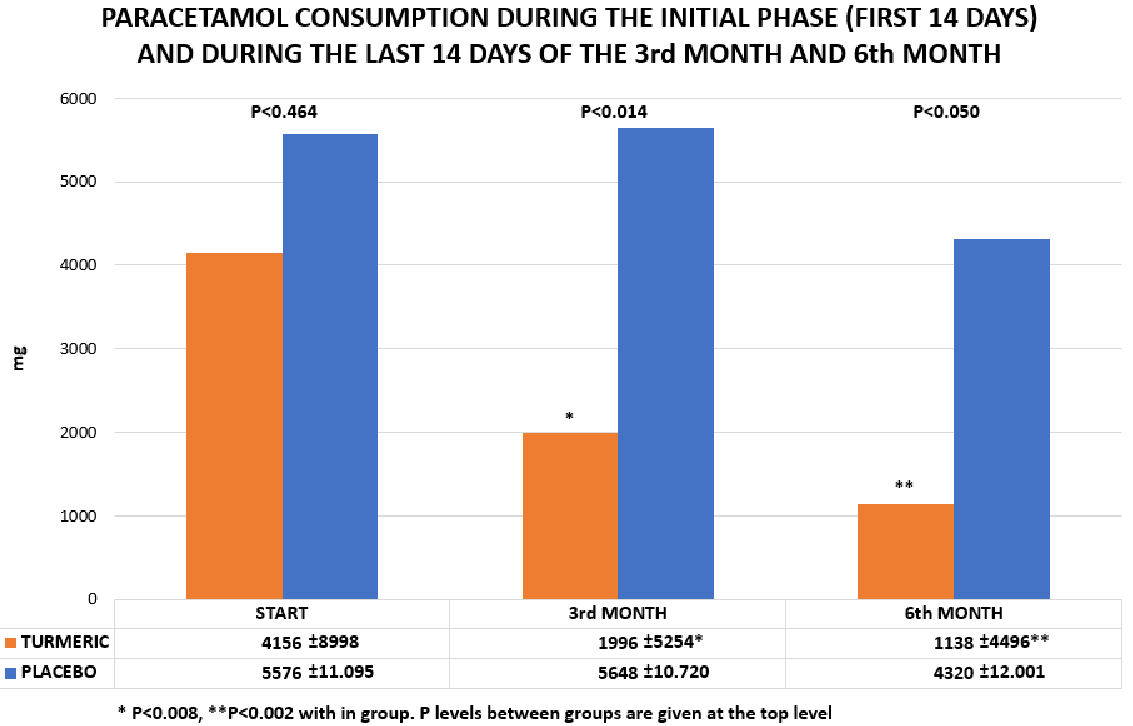
Figure 5 The consumption of paracetamol in the group of volunteers treated White Japanese Turmeric (orange) and placebo (blue). The columns indicate the mean consumption of the volunteers during the first 14 days of treatment and the last 14 days of treatment of the 3rd and 6th month, respectively.
A similar pattern was observed for NSAIDs. The initial level in the actively treated group was 355 +/- 890 mg declining to 128 +/- 769 after 6 months (p<0.006). By comparison, the corresponding values in the placebo group was initial level 568+/- 2.464 which increased to 728 +/- 3036 after 6 months. The p value for comparing the groups was p<0.0171 for differences in NSAID utilization. These data are from ITT analyses, and they are supported by per protocol data.
The consumption of rescue medication was also evaluated in the per protocol population as the number of volunteers taking rescue medication initially and after 6 months. Of the 40 volunteers, in the actively treated group, who completed all 6 months of treatment, 18 were initially on paracetamol. By the end of the trial only 6 were using paracetamol. The corresponding figures for NSAIDs was initially 10, which reduced to only 2 NSAID consumers, by the end of the trial. These changes are statistically significant (p<0.01 and p<0.025), respectively. In summary, more than 2 out of three volunteers stopped taking rescue medication as the result of treatment with Japanese White Turmeric. In the per protocol placebo treatment group, there were 13 out of 27 volunteers initially taking paracetamol, while 2 study participants were on NSAIDs. After 6 months treatment there were still 12 volunteers on paracetamol and 3 on NSAIDs. In brief, placebo hardly caused any change in the consumption of rescue medication in the placebo group (p<0.950).
Mood, general wellbeing and energy recorded in diaries
There were no indications of any effects of either Turmeric or placebo treatments to mood or general wellbeing in this study. (Data not shown). There were some interesting trends when looking at energy as Turmeric significantly improves energy (p<0.006) when evaluated within the group. No such statistically significant alteration was observed during placebo treatment (p<0.090). However, there were not statistically significant differences when comparing the two groups. (Data not shown).
Summary of findings
The overall impression of this clinical trial is that White Japanese Turmeric (WJT) seems to improve symptoms of osteoarthritis and reduce the consumption of rescue medication in patients with osteoarthritis (OA). This is evidenced from the data after three months treatment and onward indicating that active treatment was superior to placebo when testing pain on a numerical axis, the activity of daily living (ADL), patients global assessment of disease severity (PGAD) and the evaluation of how patients felt treatment worked (HTW) and by the more than 50% reduction in the consumption of paracetamol observed in the actively treated group (details in Tables 3 and 4 and in Figure 5). After six month of treatment the results seems even more convincing as WOMAC pain at this time point was also statistically significant superior to placebo. However, the six-month data should be taken with some precaution as at that time the number of volunteers in each group was not any longer sufficient to create the same power as observed after 3 months. For details see Figures 3-5 and Tables 2-4.
Comparison with previous findings
The present data is supported by the open label study17 which showed about 50% pain reduction with white turmeric after two months of treatment. It is of interest to note that in the present trial, white turmeric and placebo resulted in significant pain reductions already one month into the study periods. This positive impact remained for both treatments for the first 3 months of treatment. Such “improvements” from placebo treatments have earlier been reported in a meta-analysis of effect studies concerning treatments for osteoarthritis.21 However, after 6 months the impact from placebo has vanished, such that the pain-reducing effects in the two study groups are now significantly different from each other (p<0.041). Therefore, it may be that it takes up to 6 months treatment to observe the genuine differences between active treatment and placebo when considering pain-reduction in osteoarthritis. This should be born in mind, as many studies on pain in osteoarthritis are only designed to last for 2 or 3 months. From our observations, any conclusions made from such studies may be misleading, as our data on WOMAC pain after 3 months would not have allowed us to vote any further in favour of Japanese White Turmeric. Indeed, after 1, 2 or 3 months of the study, our conclusions would have been, that the Japanese White Turmeric does not work any better on pain than does placebo, when using WOMAC visual analogue scales.
The reduction of WOMAC pain as the result of white turmeric treatment, was further supported by additional pain scores taken upon inclusion and again after 3 and 6 months, respectively using a numerical scale focusing on the worst experienced pain during the last 7 days (details in Tables 4a and 4b). Using a numerical scale from 0-10 (10 worst possible) we were able to distinguish between active and placebo treatment after already 3 months indicating that possibly visual analogue scales (WOMAC) and numerical scales used together on the same group of volunteers can give us a wider impression of the sensation of pain than each scale alone.
Secondary effect variables like WOMAC stiffness and patients’ evaluation of disease severity (PGAD) and how the volunteers felt that “treatment was working” (HTW) followed the same pattern as outlined for “over all pain on a numerical axis” (details in Tables 3a and 3b). In line with the reduction in pain, a statistically significant drop of more than 50% was observed in the consumption of rescue medication with Turmeric treatment. This should be compared to the modest improvement in the consumption of such medication observed in the placebo group (Figure 5). Indeed, some herbal remedies also including rose-hip may have the advantage to lover the consumption of rescue medication16 and even improve the quality of cartilage.15,17
Implications for clinical practices
From the present data, it is not possible to estimate the true pain reducing impact from white turmeric, as patients in the actively treated group reduced their consumption of pain killers as described above. Indeed in the actively treated group, two out of three who initially used paracetamol had dropped the rescue medication after six month in the clinical trial and a similar pattern was observed for NSAID. One might suggest that if the consumption of pain killers had been kept constant in the two treatment groups, during the whole entire trial, the impact from turmeric on pain and other symptoms from OA, might have been even more pronounced.
However, it is important to emphasize the advantages to OA patients in that the consumption of paracetamol and NSAIDs were reduced as observed in this study, since the pain killers all have serious side effects which include liver and kidney damage and haemorrhagic stroke.2-4,6,7,22
The JWT used in this trial is very poor in curcumin (Figure 1)17 normally ranked as the most active ingredient in turmeric,23,24 but also known to cause side effects like bleeding25 and iron deficit.26 The active ingredient in JWT is labdane di-terpene, nearly undetectable in yellow turmeric (Figure 1) known as an anti-inflammatory agent which inhibits the formation of leukotrienes and improve the formation of cartilage in animal models14,17 (Personal communication: Professor Koichiro Komai, Kindal University, Japan).
The product has been on the market in Japan for 10 years and has not been reported to cause side effects. This is in agreement with our findings as the only side effect reported was milder skin rashes, which was equally reported in both groups.
Strengths and limitations
This study is the first placebo controlled, double-blind, randomized multi-centre study to be reported on Japanese White Turmeric. Different to the reported open study17 this study offers more accurate and reliable evidence on the efficacy and safety to treat OA patients with JWT owing to a rigorous design with predetermined sample size a protocol approved by the Ethics committee and blinding of patients, nurses and doctors. Clinical trials on symptoms of osteoarthritis are often based on an evaluation of symptom scores only. In our study the volunteers were also advised to use diaries regarding their consumption of rescue medication by simply giving the consumption of tablets per day in a diary. This measurement can be a more solid marker of how a disease is progressing than simple numerical or visual analogue scales on symptoms like pain and stiffness.
There are, however, limitations in our study. Possibly because our volunteers really had to fill in a lot of papers every day we lost many of our volunteers after 3 month. The main reason for leaving the study after three month was simply either that treatment did not work or there was too much work with the diaries. As 22 volunteers on placebo and 12 on active treatment left the trial after 3 month, our power calculation for the last part of the study is much weaker. Our intension was that there at least should be 50 volunteers in each group after 6 month of treatment. This was more or less the situation after 3 month where we have 52 on active and 49 on placebo treatment and several of our variables including the consumption of rescue medication are statistically significant after 3 month. But after 6 month there were only 27 volunteers in the placebo group and 40 in the actively treated group, leaving us in a weaker position, when we are discussing the final outcome.
Balancing benefits and harms indicates that White Japanese Turmeric containing nearly no curcumin but rich in labdane di-terpene can reduce symptoms of osteoarthritis including pain and at the same time reduce the consumption of rescue medication like paracetamol and NSAIDs dramatically, without causing side effects. As the consumption of paracetamol and NSAIDs represents a threat to our society because of well-known side effects the present data is of clinical interest. Our data may indicate that a clinical trial on osteoarthritis should possibly run for more than three months if we want to estimate the true impact from placebo. And using both numerical scales and visual analogue scales for pain measurements may further improve our insight. If more than 3-months treatment is needed to estimate the true difference of a herbal remedy and of placebo on symptom scores of patients with osteoarthritis many studies on OA which have been running for only 2-3 month can be misleading. The present study cannot stand on its own, and deserves further and better powered follow-up studies, which we have now documented should run for more than 3 months.
The authors acknowledge the nurses Eriksen E, Tulstrup B, Henriksen L and Secretary, Mona C for excellent technical assistance.
The authors declare that there have no conflicts of interest associated with this publication.
Kaj Winther was the initiator of this trial, wrote the protocol for the ethical committee and submitted for Clinical trial gov. All researchers participated in the practical management of the trial and in entering data. All authors participated in data analysis and in writing the paper.
Veritas Ltd supplied capsules containing active treatment or placebo and paid technicians for practical work and for the analysis of blood samples.

©2024 Winther, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.