International Journal of
eISSN: 2381-1803


Research Article Volume 8 Issue 5
1Department of Human Physiology, Federal University of Technology Owerri, Nigeria
2Department of Physiology, Imo State University Owerri, Nigeria
3Genesis Health Care, MD 21286 United States of America, USA
Correspondence: Agbai Emmanuel Onuka, Department of Physiology, School of Basic Medical Sciences, Federal University of Technology Owerri, Imo State, Nigeria, Tel +2348035976525
Received: August 22, 2017 | Published: September 13, 2017
Citation: Onuka AE, Okechukwu NC, Maxine KM (2017) A Comparative Study between Xylopia aethiopica Dried Fruit Extract and Ibuprofen Inhibiting Effects on Some Reproductive Hormones Irrespective of the Estrous Cycle. Int J Complement Alt Med 8(5): 00274. DOI: 10.15406/ijcam.2017.08.00274
Ibuprofen is often used for contraception, therefore, Xylopia aethiopica dried fruit extract and ibuprofen were tested on follicle-stimulating hormone (FSH), luteinizing hormone (LH), glucocorticoid, progesterone and estrogen levels in adult female rats in order to determine whether Xylopia aethiopica dried fruit extract can be used for contraception. Twenty one rats were randomly divided into 3 groups (n = 7) comprising of control group A, experimental Group B and C. The experimental Group B received 180 mg/kg of ibuprofen daily and experimental Group C received 300 mg/kg of Xylopia aethiopica dried fruit extract. All administration of drug and extract was done via oral route and lasted for 14 days independent of estrous cycle. Using Enzyme-linked Immunosorbent Assay the serum FSH, LH, glucocorticoid, progesterone and estrogen in both extract and ibuprofen treated rats were measured. Results showed that ibuprofen caused significant reduction of FSH (P<0.05). The extract did not cause significant difference in the serum FSH level. Both ibuprofen and extract caused significant reduction (P<0.05) in the serum LH levels. Both ibuprofen and extract caused significant elevation (P< 0.05) in glucocorticoid level. However, the extract caused significant reduction (P<0.05) in the serum progesterone and estrogen levels respectively compared to ibuprofen. In that sense, Xylopia aethiopica dried fruit extract acted as a more potent contraceptive, inhibitor of ovulatory hormones than ibuprofen.
Keywords: xylopia aethiopica, ibuprofen, follicle-stimulating hormone, luteinizing hormone, glucocorticoid, progesterone, estrogen
FSH, Follicle-Stimulating Hormone; LH, Luteinizing Hormone; Nsaids, Non-Steroidal Anti-Inflammatory Drugs; ELISA, Enzyme-Linked Immunosorbent Assay; SEM, Standard Error of Mean; ANOVA, Analyzed Using One-Way Analysis of Variance
Ibuprofen is often used for contraception; therefore, Xylopia aethiopica dried fruit extract and ibuprofen were tested on follicle-stimulating hormone (FSH), luteinizing hormone (LH), glucocorticoid, and progesterone and estrogen levels in adult female rats in order to determine whether Xylopia aethiopica dried fruit extract can be used in contraception. Twenty one rats were randomly divided into 3 groups (n = 7) comprising of control group A, experimental Group B and C. The experimental Group B received 180 mg/kg of ibuprofen daily and experimental Group C received 300 mg/kg of Xylopia aethiopica dried fruit extract. All administration of drug and extract was done via oral route and lasted for 14 days. Using Enzyme-linked Immunosorbent Assay the serum FSH, LH, glucocorticoid, progesterone and estrogen in both extract and ibuprofen treated rats were measured. Results showed that ibuprofen caused significant reduction of FSH (P < 0.05). The extract did not cause significant difference in the serum FSH level. Both ibuprofen and extract caused significant reduction (P < 0.05) in the serum LH levels. Both ibuprofen and extract caused significant elevation (P < 0.05) in glucocorticoid level. However, the extract caused significant reduction (P < 0.05) in the serum progesterone and estrogen levels respectively compared to ibuprofen. In that sense, Xylopia aethiopica dried fruit extract acted as a potent contraceptive, inhibiting the hormones critical in initiating ovulation.
In females reproductive hormones play an important role in extensive concerted coordination of reproductive cycle and these hormones include hormones of the hypothalamic-pituitary-ovarian (HPO) axis. Study has shown that alteration in the HPO axis leads to disruption of ovarian functions.1 Non-steroidal anti-inflammatory drugs such as ibuprofen and mefenamic acids have been shown to have high endocrine disruption potentials.2 Ibuprofen is a (2RS)-1.4-(2-methyl propyl phenyl propionic acid that is commonly and frequently used non-steroidal anti-inflammatory drugs (NSAIDs) 3. Ibuprofen is often used for contraception in the relief of pain from dysmenorrhea in women.4 Studies in vivo and in vitro have demonstrated that chronic administration of ibuprofen caused significant reduction in ovulation rates of female mouse and damaged fertility of male mouse.5, delayed timing of ovulation in humans.6, and selectively blocks estrogen-mediated receptors in rats.7 Moreover, the use of synthetic steroidal contraceptives has proven unsafe to the health of the user as they pose risk of side effects.8-10
In Africa, traditional methods of contraception are on the increase and have been increasingly sought for in herbal plants because herbs are effective, cheap and safe.11. These herbal plants have proven clinical relevance in contraception because of their active phytochemical contents. In Nigeria, several studies have shown that plants such as Mucuna soloanei and Senna occidentalisare used as contraceptives by indigenous ladies in south-south Nigeria.12, while Newbouldia laevis Seem., Ricinus communis L., Azadirachta indica. Securidaca longepedunculata and Vernonia amygdalina are frequently used for contraceptives in northern Nigeria.13 In southwest Nigeria, several plants such as Aframonium melegueta, Carica papaya and Tetrapleura tetraptera are combined and often applied on a metal ring or rubbed on fresh skin incision to serve as male contraceptives.14 In northwest Nigeria, herbs like Acacia polyacantha, Crotalaria spp, Dalbergia saxatilis, and Erythrina senegalensis are used for contraceptives.15. These herbal plants alter reproductive hormones, therefore, disrupt the reproductive cycle. Recent studies have demonstrated that Tetrapleura tetraptera and Piper guineense altered luteinizing hormone secretion during proestrus phase of the estrous cycle in rats.16,17 Meanwhile, in ancient times, women drank extracted water from Xylopia aethiopica as a natural method of contraception that became part of Igbo folklore and this led to the present research work. The plant, Xylopia aethiopica commonly known as "Uda" in Igbo language, "Eeru alamo" in Yoruba language and "Chimba" in Hausa language is a tropical evergreen, aromatic plant that grows commonly in wet swampy soils and forest zones of West Africa characterized with a slim tall tree of about 15 - 30 m tall and 0.6 - 0.7 m in diameter, straight stem, slightly stripped or smooth bark with consistent pepperish aroma with dry brown, corrugated in shape with pods containing 4 - 9 kidney shaped peppery seeds about 0.05 m in length.18,19
However, antifertility effect of Xylopia aethiopica have been demonstrated in male rats.20,21 In another study unpublished, we demonstrated that Xylopia aethiopica dried fruit extract caused alteration in luteinizing hormone (LH), follicle-stimulating hormone (FSH), and glucocorticoid hormone during proestrus phase of clomiphene-treated rats. Because Xylopia aethiopica have analgesic properties and can be used in the management of pain disorders similar to ibuprofen.4,22, therefore, the present study compared the inhibiting effects of ibuprofen and Xylopia aethiopica dried fruit extracts on the serum levels of FSH, LH, glucocorticoid, estrogen and progesterone in order to determine the contraceptive potential of Xylopia aethiopica dried fruit extract independent of the estrous cycle in adult female Wistar rats.
Animals
Twenty one female albino wistar rats weighing (180 - 250g) were obtained from the Animal House, Department of Pharmacology, College of Medicine and Health Sciences, University of Port Harcourt, Nigeria. They were transported and housed in the Animal House, Department of Human Physiology, Faculty of Basic Medical Sciences, Madonna University, Elele campus, Nigeria. The rats were kept under standard laboratory condition and fed with normal rat feed and water ad libitum. The animals were kept in standard cages (Henan, China) and allowed to acclimatize for two weeks under a room temperature between 27 °C and 29 °C.
Ethics: The research protocol was approved by the Ethics committee of the Faculty of Basic Medical Sciences, Madonna University Nigeria. All the animals received humane care according to the criteria outlined in the Guide for the Care and the Use of Laboratory Animals prepared by the National Academy Science and published by the National Institute of Health.23
Experimental design
Twenty one rats irrespective of their estrous cycle were divided into four groups (n = 7): group A (normal control) received rat feed and water, Group B received high dose oral administration of 180 mg/kg of ibuprofen daily (Bristol, UK) as published in Pfizer Safety Data Sheet.24 Group C rats received oral administration of 300 mg/kg of Xylopia aethiopica dried fruits extract daily according to method described by Agbai et al.25,26 The administration of drug and extract lasted for 14 days.
Extraction of plant
The dried fruits of Xylopia aethiopica were purchased from the Afor Ogbe market in Ahiazu Mbaise, Imo State, Nigeria. They were authenticated in the Department of Pharmacognosy, Faculty of Pharmaceutical Sciences, Madonna University, Elele, Nigeria, and a voucher specimen number MUE/PGSY/011. The dried fruits were pounded in a mortar into a coarse powdered form. 150 g of the coarse form was weighed out and extracted with 200 ml of ethanol (Sigma Aldrich, USA) using extraction maceration method as described by Sukhdev et al.27 for 24 hours in an air tight container. Using a vacuum rotary evaporator, the ethanol filtrate was concentrated at a low temperature, under reduced pressure and this yielded 21 g jelly-like pungent oily extract. All measurements were done using laboratory balanced (Grainger, UK). 21 g of the oily extract was further dissolved in 5 ml of ethanol and was subsequently stored in a refrigerator of temperature 4 °C. 1.5 g of the freshly prepared solution was dissolved in distilled water daily for 14 days.
Drug preparation
One packet (84 tablets per pack, 400 mg per tablet) of Ibuprofen (Bristol, UK) was purchased over the counter at Orchard Pharmacy, Owerri, Imo State, and ground into a fine powdered form in a mortar and extracted with ethanol to remove excipients using whatman paper (no. 1). The filtrate was administered as ibuprofen. All oral administration was carefully performed using oral gavage.
Hormone measurement
At the end of experiment, twenty rats were anesthetized in a glass chamber one after another with urethane soaked in cotton wicks repeated after three exposures. Blood was sucked up from the heart using 5 ml syringe after cardiac puncture and pushed out into a well labeled EDTA bottles to prevent blood coagulation. FSH, LH, glucocorticoid, progesterone and estrogen were measured using Enzyme-linked Immunosorbent Assay (ELISA) method.
Statistical analysis
Results were expressed as mean ± Standard Error of Mean (SEM). Statistical significance of difference observed between control and experimental groups was analyzed using one-way Analysis of Variance (ANOVA). Any significant ANOVA was further analyzed by Tukey's post hoc test. P values < 0.05 were considered statistically significant.
Results in Table 1 showed that serum FSH level in extract-treated Group C (0.29 ± 0.01 miU/ml) was not statistically significant (P > 0.05) compared with normal control group A (0.37 ± 0.10 miU/ml). However, serum FSH level in ibuprofen-treated Group B rats (0.10 ± 0.01 miU/ml) was significantly reduced (P < 0.05) compared to Groups A and C rats.
|
FSH (mIU/ml) |
LH(mIU/ml) |
Estrogen(pg/ml) |
Progesterone (ng/dl) |
Glucocorticoid (ng/ml) |
|
|
Control |
0.37±0.10 |
1.21±0.60 |
30.00±1.02 |
17.90±0.40 |
70.80±0.85 |
|
Group B |
0.10±0.01 |
0.34±0.06 |
32.05±1.09 |
21.00±0.50 |
80.90±1.39 |
|
Group C |
0.29±0.01 |
0.31±0.03 |
18.00±0.51 |
11.59±0.54 |
87.90±0.91 |
Table 1 The effects of ibuprofen and Xylopia aethiopica extract on some reproductive hormones
Values = mean ± SEM. *P value significant < 0.05
Serum LH level was significantly reduced (P < 0.05) in Group B (0.34 ± 0.06 miU/ml) and Group C (0.31 ± 0.03 miU/ml) compared to control group A (1.21 ± 0.60 miU/ml). The serum LH level was not significant (P > 0.05) in Group B compared with Groups C rats.
Table 1 also showed statistically significant reduction (P < 0.05) in serum estrogen and progesterone levels in extract-treated Group C rats (18.00 ± 0.51pg/ml, 11.59 ± 0.54ng/ml) compared to control group A (30.00 ± 1.02 pg/ml, 17.90 ± 0.40ng/ml) and Group B (32.05 ± 1.09pg/ml, 21.00 ± 0.50ng/ml) respectively.
Serum glucocorticoid level was significantly elevated (P < 0.05) in Groups B and C (80.9 ± 1.39, and 87.9 ± 0.91ng/ml) compared to control group A (70.8 ± 0.85ng/ml). Serum glucocorticoid level was not significant (P > 0.05) in Group B compared with Group C.
The present study showed that 300 mg/kg of the extract did not cause significant change in the serum FSH level. Several studies have shown that pattern of action of the extract on FSH secretion is dependent on dose concentration in male rats. It has been shown that 80mg/kg of Xylopia aethiopica dried fruit extract caused significant reduction in FSH level in male Wistar rats.28 However, the dose-dependent action of the extract was clarified by Abass who demonstrated that low dose of the extract (30 mg/kg) caused significant increase in FSH level and high dose of the extract (between 100 and 300 mg/kg) caused significant reduction in FSH level in male rats.29 These reports implied that the action of the extract is dose-dependent. However, our laboratory study (unpublished) demonstrated that 300 mg/kg of the extract elevated FSH level during proestrus and estrus in female rats. Based on this report, it can be suggested that the action of Xylopia aethiopica extract on FSH secretion is not only dose-dependent but cycle-dependent. Studies have shown that FSH secretion is pulsatile, but the mean level of FSH secretion remains constant in male and varies during menstrual cycle in women with increased number of secretory burst during late follicular phase of the menstrual cycle.30,31 Moreover, Onyebuagu et al. revealed that 500 mg/kg of Xylopia aethiopica extract did not cause significant change in FSH level of female rats.32 in consistent with our findings and confirmed that extract-mediated FSH secretion is dependent on the estrous cycle in rats.
Moreover, ibuprofen inhibits cyclooxygenase enzymes responsible for prostaglandin synthesis.33 Studies have shown that prostaglandins PGF2α and PGE2 mediate direct effect on the hypothalamic-pituitary axis causing FSH peak.34,35, suggesting that since ibuprofen could inhibit cyclooxygenase enzyme that is the dominant source prostaglandins, thereby attenuating prostaglandin release causing the reduction of FSH in the present study. The significant reduction in FSH level also corroborated with our previous work that showed significant reduction in FSH level in ibuprofen-treated rats.35
Figure 1 showed that extract and ibuprofen caused significant reduction in the serum LH level respectively. The extract reduction of LH corroborated with our previous work that revealed Xylopia aethiopica extract caused significant reduction in serum LH level during proestrus and estrus phases. Moreover, saponins have been shown to inhibit LH release from the gonadotropes.36, and quantitative phytochemical evaluation of Xylopia aethiopica dried fruit showed the presence of significant quantity of saponin in the fruit.37 As aforementioned, LH peaks secondary to stimulation of gonadotropin-releasing hormone release after injection of prostaglandin PGF2α in chlorpromazine-induced anovulation.34,38, also suggesting that blockade of prostaglandins by ibuprofen could result in the reduction of LH level. The present study indicated that both Xylopia aethiopica extract and ibuprofen caused significant reduction in LH level suggesting the possibility the extract in the impairment of ovulation.
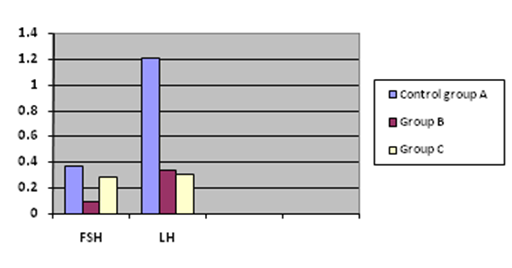
Figure 1 The comparative effect of ibuprofen and Xylopia aethiopica on follicle-stimulating hormone (FSH) and luteinizing hormone (LH).
Figure 2a showed that Xylopia aethiopica fruit extract caused significant reduction in estrogen and progesterone level compared to ibuprofen and normal control. Moreover, estrogen is primarily synthesized in ovaries, corpus luteum and placenta and catalyzed by aromatase enzyme that is critical in the last step of estrogen synthesis.39
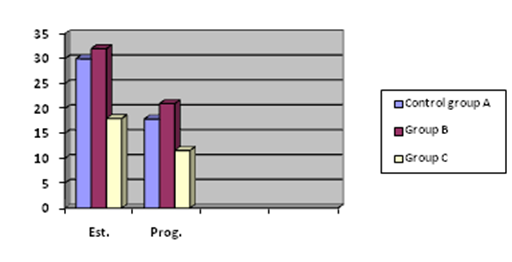
Figure 2a The comparative effect of ibuprofen and Xylopia aethiopica on estrogen (Est) and progesterone (Prog).
In the context of this study, ovaries are central in the synthesis of estrogen and could be altered by the extract. During the process of folliculogenesis, thecal and granulosa cells are involved in the estrogen synthesis. Theca cells cannot directly produce estrogen and therefore in growing follicles androgens are released from the thecal cells and transported to the granulosa cells where P450 aromatase enzyme converts androgens to estrone and 17-beta estradiol.39,40. In addition to saponin as phytochemical content of Xylopia aethiopica, flavonoids are one the major phytochemical contents of the Xylopia aethiopica dried fruit extract.41 Studies have shown that saponins and flavonoids inhibit aromatase enzyme in human preadipocyte.42-44 and inhibition of aromatase enzyme has been shown to reduce estrogen production throughout the body to nearly undetectable levels.45 To a large extent, estrogen from androgen conversion contributes to the estrogen pool. Moreover androgen production from the thecal cells is largely under the control of LH from the pituitary.46 Therefore, the decrease in LH observed in the Xylopia aethiopica extract treated Group C rats could impair androgen production and inhibit the conversion of androgens into estrogens in the granulosa cells resulting in the reduction in estrogen level.
Moreover, corpus luteum is a dynamic endocrine gland within the ovary and originates from the thecal and granulosa cells following breakdown of the basal lamina immediately prior to follicle rupture.47 Corpus luteum differentiates from the mature follicle after ovulation and the corpus luteal cells are the principal secretors of progesterone that is dependent on the LH.48 More so, it has been shown that LH receptor is maintained throughout the functional lifespan of the corpus luteum.48 Because the extract caused significant reduction of LH, it can be stated that corpus luteum formation and secretion of progesterone will be impaired resulting in the reduction of progesterone.
Results in Figure 2b showed that both extract and ibuprofen caused significant elevation of glucocorticoid level. The glucocorticoid is produced by neuroendocrine pathway that consists of hypothalamus, pituitary and adrenal glands. The hypothalamus secretes corticotrophin-releasing hormone, which stimulates the synthesis and secretion of adrenocorticotropic hormone into the circulation. Adrenocorticotropic hormone subsequently induces the secretion of glucocorticoid from the zona fasciculata of the adrenal gland. Although glucocorticoids are known to have a strong anti-inflammatory properties similar to ibuprofen, but studies have demonstrated that NSAIDs have an effect on hypothalamic-pituitary adrenal axis.49-51 via inhibition of cyclooxygenase enzyme.52 However, study on the ulcerogenic gastrointestinal side effects of NSAIDs has shown that NSAIDs induced an increase in glucocorticoid production.53 corroborating with the results of the present study.
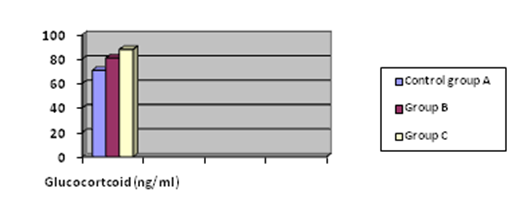
Figure 2b The comparative effect of ibuprofen and Xylopia aethiopica on estrogen (Est) and progesterone (Prog).
Moreover, study has shown that 30-300 mg/kg of Xylopia aethiopica dried fruit possessed analgesic properties.22 Since there is a paucity of literature on the effect Xylopia aethiopica dried fruit extract, it is therefore suggested that the significant elevation of glucocorticoid induced by the extract in the present study could follow the same pattern of mechanism of action of ibuprofen.
Results revealed that Xylopia aethiopica dried fruit extract caused significant reduction in the serum estrogen and progesterone level compared to ibuprofen although both extract and ibuprofen caused significant reduction in the serum LH levels as well as significant increase in serum glucocorticoid independent of the rats' estrous cycle suggested that both could prevent induction ovulation due to inhibition of LH secretion. However, the marked reduction of estrogen and progesterone by the extract further confirmed the inhibition of ovulation and absence of corpus luteum and can be used for contraception.
The authors want to gratefully thank Mr Raymond Okonkwo for his technical assistance. Furthermore, we wish to thank Professor A. C. Ugwu for his guidance and critical examination of this work.
The authors have none to declare.

©2017 Onuka, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.