International Journal of
eISSN: 2381-1803


Opinion Volume 11 Issue 1
Purdue Research Park, USA
Correspondence: Daniel Sliva, DSTest Laboratories, Purdue Research Park, 5225 Exploration Drive Indianapolis, IN 46241, USA, Tel 3173 7937 02
Received: February 21, 2018 | Published: February 28, 2018
Citation: Sliva D. Personalized cognitive improvement by dietary supplements. Int J Complement Alt Med. 2018;11(1): 00358. DOI: 10.15406/ijcam.2018.11.00358
Cognition functions, including attention, memory, reasoning, and language are affected by several diseases including Alzheimer’s disease, Parkinson’s disease and other neurological disorders.1 Specific nootropic drugs that may improve the cognitive function in patients with memory disorders may also increase performances in healthy people.2 Moreover, natural compounds and supplements demonstrated potential adjunctive therapeutic effects on various neurodegenerative diseases.3 The global market for cognition-enhancing supplements exceeded 1 billion USD in 2015.4 Some patients are fighting age-related cognitive decline, whereas others are focused on enhancement of their mental performance. Since the market is flooded with a plethora of cognitive-enhancing supplements, and dietary supplements are not regulated by the FDA, the effectiveness of some of these supplements may be questionable. Turmeric/curcumin and green tea extract are among the most popular dietary supplements used mainly for their anti-oxidative activities and their effect on the immune system.
Several recent clinical studies have demonstrated a positive effect of turmeric/curcumin on a variety of medical conditions. Curcumin supplementation improved artery endothelial function and reduced oxidative stress, which is associated with cardiovascular disease, in healthy middle-aged and older adults.5 Curcumin also demonstrated anti-inflammatory activity by significant reduction of serum atherosclerotic low-density lipoprotein levels in patients with mild chronic obstructive pulmonary disease (COPD).6 Curcuminoids significantly prevented the development of diabetes in a prediabetic group,7 and markedly increased anti-oxidant activity in the blood of patients with type II diabetes.8 Curcumin significantly reduced neuropathic pain,9 significantly decreased pain in aged patients with knee osteoarthritis,10 and decreased pain and prevented muscle injury after running.11,12 Supplementation of curcuminoids to standard antidepressants showed a significant reduction of anxiety and depression in patients with major depressive disorder.13 Moreover, curcumin extracts significantly improved depressive symptoms and demonstrated anxiolytic effects in patients with atypical depression.14 The positive effects on cognition was confirmed in several clinical trials. A randomized, placebo-controlled, double-blind study demonstrated that curcumin prevented cognitive decline in older adults.15 Another study showed significant improvement of sustained attention, working memory tasks, and mood after curcumin treatment.16 Moreover, recent studies with mouse models of Alzheimer disease found that curcumin ameliorates cognitive decline and improves synaptic functions. Ongoing studies of the effect of curcumin on Alzheimer disease patients are currently being assessed.17 On the molecular level, curcumin inhibits the formation and promotes the disaggregation of amyloid-β plaques, attenuates the hyperphosphorylation of tau and enhances its clearance, binds copper, lowers cholesterol, modifies microglial activity, inhibits acetylcholinesterase, mediates the insulin signaling pathway, and has antioxidant and anti-inflammatory activity.18
Several clinical studies found healthy effects of green tea in humans. Two independent double-blind, placebo-controlled clinical trials demonstrated that green tea extract (GTE) significantly reduced blood pressure in obese pre-hypertensive women,19 and obese hypertensive patients.20 GTE reduced pockets of inflammation in patients with chronic periodontitis,21 and significantly reduced body weight and waist circumference in obese women.22
Neuroimaging analysis (functional magnetic resonance imaging, fMRI) of healthy volunteers showed that GTE activated the dorsolateral prefrontal cortex (DLPFC), a key area that mediates working memory in the human brain.23 A randomized, double-blind, placebo-controlled study using a combination of GTE and l-theanine demonstrated improved memory and attention in subjects with mild cognitive impairment.24 A double-blind, placebo-controlled trial demonstrated that GTE significantly increased visual recognition memory in young adults with Down syndrome.25 Mechanistically, GTE induction of neuronal plasticity resulted in cognitive improvement.26 In addition to anti-oxidative and anti-inflammatory activities, GTE may improve cognitive function by the inhibition of arterial stiffness.27 Although the above described clinical studies demonstrated significant improvement of cognition by characterized curcumin and green tea extract, there are several concerns that need to be addressed.
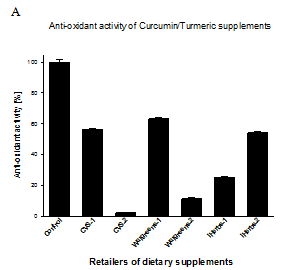
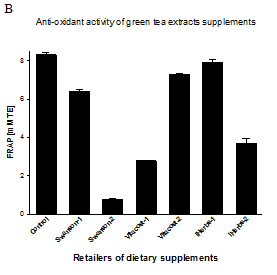
Figure 1 Anti-oxidant activity of Curcumin/Turmeric and green tea extract dietary supplements. (A) Anti-oxidant activity of Curcumin/Turmeric extracts is expressed as percentage of activity compared to the same amount of control pure curcumin. (B) Anti-oxidant activity of green tea extracts is expressed as Trolox equivalent (mM TE) compared to the same amount of control pure catechin EGCG. The data are averages from three independent experiments±SD. Tested dietary supplements were obtained from CVS Pharmacy, Walgreens, Swanson, Vitacost and iHerbs retailers.
In summary, an improvement of cognitive functions by using dietary supplements is a complex process. Among a plethora of dietary supplements marketed for cognitive improvement, we focused on curcumin/turmeric and green tea extracts. Although clinical studies are showing promising results, a more sophisticated and personalized approach using supplements targeting specific genes is necessary. Recent international genome-wide association study (GWAS), using transcriptome-wide and epigenome-wide analysis, identified novel drug targets for several potential nootropic mechanisms [30]. Development of a novel nutrigenomic approach for targeted individual cognitive improvement by dietary supplements is the next step in the enhancement of human potential.
None
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.

©2018 Sliva. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.