International Journal of
eISSN: 2381-1803


Research Article Volume 9 Issue 6
Department of Acupuncture, Harvard Medical School, USA
Correspondence: Nadia Volf, Department of Acupuncture, Harvard Medical School, Lecturer in the International Structural Acupuncture Course at Harvard Medical School (USA), Paris 75008, France, Tel (33) 608861094
Received: October 27, 2017 | Published: December 11, 2017
Citation: Volf N, Ferdman L (2017) The Mechanisms of Immunomodulation in Acupuncture. Int J Complement Alt Med 9(6): 00320 DOI: 10.15406/ijcam.2017.09.00320
The heart generates the strongest and most extensive rhythmic electromagnetic field in the body. As all cells throughout the body are oriented according to the magnetic vector of the heart, the heart has a powerful influence on everybody process. The activity of immune cells is closely connected to their polarity: when immune cells become depolarized, they are deactivated as a result.
We hypothesize that the heart’s electromagnetic signals are distributed throughout the body via the network of acupuncture meridians, similar to the manner in which the heart propels blood throughout the body via blood vessels. The heart’s electrogmagnetic system governs the structure and organization of tissues, as well as the potential – and thus the activity – of all the body’s cells, including immune cells. This method of communication is implied in the control of cell proliferation, and its failure in a bodily organ contributes to the cancer process.
Our aim was to determine whether we could monitor the electromagnetic activity in acupuncture points (back SHU points) and record this activity with electrocardiogram or electroencephalogram. In our preliminary trial, we succeeded in recording this electromagnetic activity using electrogram to record activity in back SHU acupuncture points (also known as Associated Points, or Paravertebral Reflex points), but not in an inactive neighboring area (control or “sham” points). We found that the wave shape of electromagnetic activity recorded at SHU points was different in the presence of pathological conditions in a given internal organ, while it remained constant in SHU points representing healthy organs.
In conclusion, our preliminary research offers additional proof for the existence of a sovereign system of biological control over the organization and activity of all bodily tissues, governed by the electromagnetic field generated by the heart and distributed through the network of acupuncture meridians. Our research leads us to believe that the immunomodulating effect of acupuncture results from the restoration of tissue cell structure and cell potential. The stimulation of acupuncture points restores the receptivity of target tissues and immune cells to the heart’s electromagnetic signals, thereby restoring the cells’ polarity and functional activity.
Keywords:acupuncture, rhythmic electromagnetic field, heart’s electrogmagnetic system, shu points, immunomodulating effect, genetic pathways, bioelectrical activity
MCG: Magnetocardiogram; EKG: Electrocardiogram; EEG: Electroencephalograms
Multiple recent studies have highlighted the role of bioelectric signals in large–scale pattern formation in the body’s spatial structure and organs, and in organ phylogenesis and ontogenesis, during embryogenesis and regeneration.1–5 These signals form one layer of physiological control and work in conjunction with biochemical and genetic pathways.6–10 The bioelectrical state of cells and tissues was shown to regulate their differentiation, migration, and proliferation and plays an important role in the development of cancer.11–25 Bioelectrical activity has long been implicated in neoplasm, and recent molecular efforts have focused on ion channels as important cancer targets, and ion channel drugs as a promising therapy.26–35 The nature of these formative biological fields has been defined in modern experimental studies and theoretical constructs as electromagnetic.36,37
Many papers.38,39 have addressed the influence of electric, magnetic and electromagnetic forces in cells, tissues and the entire organism. Indeed, the activity of all cells in the body fundamentally relates to their biophysical features, which differ under healthy state and pathological conditions, and especially during the cancer process. The activity of a cell’s receptors depends on its state of polarity: depolarization is accompanied by deactivation. Specifically, the depolarization of immune cell receptors corresponds to their deactivation and inability to recognize and eliminate pathogenic cells, especially cancer cells.40,41 The disorganization and depolarization of tissue structures leads to their predisposition to be involved in pathological processes, and to the development of cancer.42
Polarization has a profound effect on the outcome of oncogenic signaling. For example, chronic activation of oncogenic c–Myc results in increased cell proliferation in cells that grow as an unorganized cell population, but has no effect in the same cells when they can form polarized 3D structures. Cellular organization into epithelial architecture maintains structural integrity and homeostasis by suppressing cell proliferation and apoptosis. Organized epithelial architecture is critical for suppression of proliferative oncogenesis, and regulates sensitivity to apoptotic oncogenic function.43
Thus, the combination of cell polarity disruption and tissue disorganization is a hallmark of advanced epithelial tumors. In normal tissue, simple epithelium comprises a monolayer of individual cells that display distinct apical–basal polarity. Cells are tightly packed and connected to each other by the apical junctional complexes, which separate apical and basolateral membrane domains. By contrast, in high–grade epithelial tumors, cells display a loss of apical–basal polarity and overall tissue disorganization.42
Cancer cells exhibit different polarity compared to cells in surrounding tissues. Cancerous tumors display altered physico–chemical parameters, including the electrochemical equilibrium of the tumor environment. Communication between cancer cells occurs via broadcast. Biophotones influence DNA emissions and structure. In cancer cells, DNA emissions become inconsistent.40
The body constantly produces an abundance of potentially cancerous cells. The role of the immune system (for example, killer T cells, and especially CD8 cytotoxic cells and macrophages) is to recognize and eliminate potentially cancerous cells. Cancer development is possible only when immune cells lose their ability to identify potentially cancerous cells, due to the desensitization and deactivation of immune cell receptors.40,41,44
The activity of immune cells and the sensitivity of their receptors is related to their state of polarity: when immune cells become depolarized, their receptors are deactivated. The question remains, what system in the body controls polarity and cell activity, including in immune cells?
Some researchers propose that acupuncture amplifies the biological electromagnetic field, explaining that the enhanced movement of stem cells by a weak electromagnetic field accounts for the results of acupuncture treatment.45
The ancient tradition of acupuncture has always attributed control of all cell activity to the heart. According to the classic ancient acupuncture text, The Yellow Emperor Classic of Medicine, “The Heart is the Lord and Master of all beings... If then the sovereign radiates, those under him will be at peace; through this the nurturing of life will yield longevity, and that from generation to generation, and the empire will radiate with a great light…”.46
In addition to the network of nervous and vascular communications, the heart communicates information to the brain and the rest of the body via the electromagnetic field. The heart generates the strongest and most extensive rhythmic electromagnetic field in the body, with a powerful influence on all body processes since all cells are oriented to the magnetic field vector of the heart. The specificity of the heart’s electromagnetic activity is well known and is widely used for diagnostic purposes, as in the case of the electrocardiogram (EKG) and magnetocardiogram (MCG).
The heart’s magnetic field, 5,000 times stronger than the magnetic field of the brain, can even be detected at a distance using a magnetometer. All of the body’s organs and cerebral rhythms are naturally synchronized with the rhythmic activity of the heart, and respond to changes in heart rate, blood pressure, emotional states and respiration. Thus, the heart produces an overall – or “sovereign” – electromagnetic field, to which the entire body is synchronized, and which directs the polarity and thus the activity of all cells, including immune cells.47
The ancient understanding of the heart’s dual facets – the visible and the invisible can be explained as two physiological functions:
The goal of this study was to explore the propagation of the heart’s electromagnetic emissions throughout the body via the acupuncture meridian network and the heart’s role in controlling cell polarity and function.
We posit that one of the mechanisms of acupuncture action, in particular its immunomodulating effect, is due to the improved sensitivity and activation of immune cells resulting from the restoration of the polarity of their receptors. As the needling of acupuncture points (which have lower electrical resistance compared to surrounding tissue) increases their electrical resistance, it activates the receptivity of immune cells to the heart’s electromagnetic signals and thus activates their polarity and functional activity.
Our first goal was as follows: since acupuncture points are frequently described as having distinct electrical properties (increased conductance and elevated electrical potential compared to adjacent non–acupuncture points).48 and if we are right that acupuncture meridians carry electromagnetic emissions generated by the heart, then we should be able to monitor the electromagnetic signal at an acupuncture point over an extended time period, as is done with electrocardiograms (EKG) and electroencephalograms (EEG).
Therefore, we attempted to record the electrical signal (electrogram) at the back SHU points and produce a visual graphic of their electric activity, using electroencephalography, where acupuncture needles, placed in acupuncture points, served as electrodes, and were connected to the EEG recorder. The second goal was to check the specificity of this electrical signal recorded from the acupuncture points. For this purpose, we compared the electrogram readings of real acupuncture points with control points (inactive areas or “sham” points).
Finally, we wanted to determine if the electrogram recording of acupuncture points on the back – the SHU points – might have diagnostic value for the state of different organs, in the same way the EKG is used for heart diagnostics, and the EEG for brain diagnostics. We chose back SHU points for the electrogram tests because they are well known as diagnostic points (points that become tender under palpation when their associated organs are malfunctioning). We compared electrograms of back SHU points in healthy volunteers and in patients with a confirmed pathological condition of an associated organ.
As in electroencephalography, the diagnostic applications were focused on the spectral component of the electrogram, that is, the type of oscillations that can be observed in electrogram signals.49 Electrogram tests were performed in 18 patients, 25–75 years old. Fifteen were healthy subjects (10 men, 5 women); 5 patients had gastric ulcer confirmed by endoscopy, and 3 patients had cardiac arrhythmias. Sterile metal acupuncture needles 0,16x25 mm were used as recording electrodes for the EEG device, as their material was conductive.
The following pairs of symmetrical points were used:
Due to the small number of subjects, acupuncture points tested, and diseases studied, our findings are preliminary. There are many sources of electrical activity in the body, including the heart, respiratory system, and skeletal muscles. We found that the waveform of the electrogram reading of each SHU point was specific and different from that of other SHU points. We observed that substantial changes in the electrographic spectrum of the SHU point electrogram reading occurred when the associated organ was diseased. This might indicate the value of taking an electrogram of SHU points for the early diagnosis of organ disease.
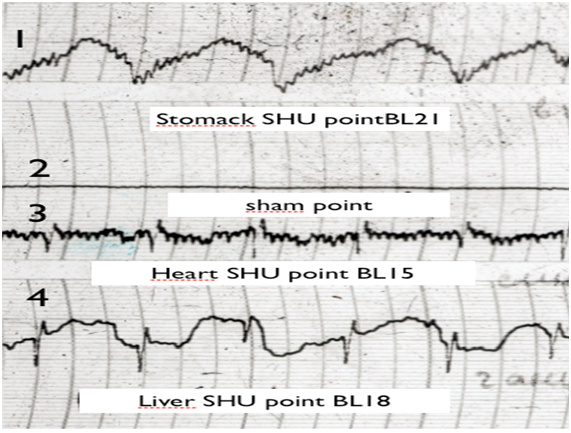
Figure 1 EG traces of sham (non active point) and SHU acupuncture points:
1: B121 (stomack SHU point);
2: Sham point;
3: B115 (Heart SHU point);
4: B118 (liver SHU point).
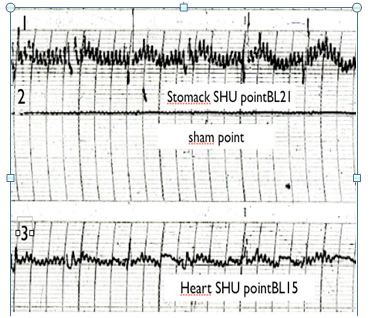
Figure 2 Patient with stomack ulcer. EG traces of 1: Stomack SHU point(Bl21), 2: sham (nonactive point) and 3: Heart SGHGU point(B115).
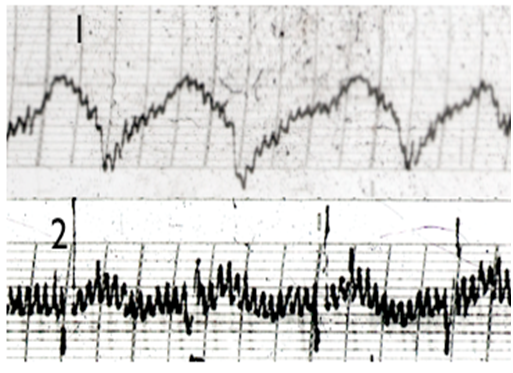
Figure 3 EG traces of Stomack SHU acupuncture points (B121): 1: Healthy person; 2: Patient with confirmed Stomach ulcer.
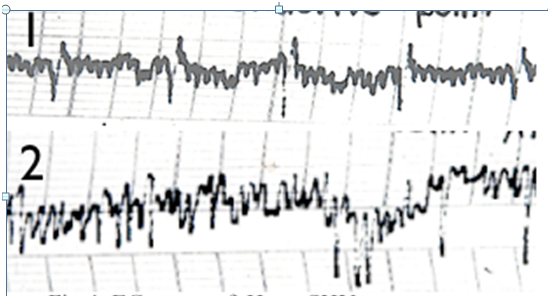
Figure 4 EG traces of Heart SHU acupuncture points (B115): 1: Healthy person; 2: Patient with cardiac arrhythmias.
In our studies, SHU points demonstrated continuous electrical activity that was absent in “sham” points. This means that acupuncture points might emit an electromagnetic field, specific to each point, which might be recordable via electrogram. We noted that the duration of electrical activity recorded at SHU points was limited to 20–25 minutes, after which electrical activity disappeared. This phenomenon probably indicates a deactivation of the acupuncture point after 20–25 minutes of stimulation. The acupuncture needles serving as electrodes in this research were placed directly in the acupuncture points, so the needles might have influenced the electrical and physiological activity of the point.
The question remains: does the electrical activity of acupuncture SHU points, as recorded by electrogram in our studies, reflect the heart’s electromagnetic field, as distributed through the network of acupuncture meridians and points? Or does it reflect specific spontaneous electrical activity of acupuncture points, which generate their own electrical activity, resulting from ionic current flowing within the specific structures of an acupuncture point? Or does it correspond to the electrical activity of the spinal nervous system, related both to an internal organ and to its associated acupuncture SHU point? We hope to answer these questions in future studies.
Acupuncture without a doubt stimulates immunity, both cellular pattern (different fractions of NK) and humoral factors (interleukins, interferons and others immunological substances).50–55 Acupuncture’s immunomodulating effect is related to the activation of Beta–endorphins in the hypothalamic centers. This activation might employ not only neurological pathways, but may also be related to electromagnetic induction, controlled by the heart, and launched by the stimulation of the acupuncture point.
From a physical standpoint, the intensity of the propagation of electromagnetic emissions depends on two things: the power of the source of these emissions, and the receptivity of tissues. The level of receptivity is conditioned by the level of the tissue’s electrical resistance: the lower the resistance, the lower the electromagnetic reception. Areas with lower resistance lose their capacity to receive the heart’s electromagnetic emissions which maintains the state of polarity and of functional activity of cell receptors. This leads to the loss of the cell’s apical–basal polarity (depolarization) and overall tissue disorganization.42 This disorganization and depolarization of tissue structures leads to the cell’s predisposition to pathological processes and to the development of cancer.42–45
From a biophysical point of view, the acupuncture points, and especially acupuncture meridians, display lower resistance than other tissues.48,56,57 Acupuncture meridians have the lowest electrical resistance (7,000–70,000 OHMs) compared to the resistance of neighboring tissues (300,000–2,000,000 OHMs). The stronger the pathological process, the lower the electrical resistance in the corresponding acupuncture meridian.
Thus, the needling (or electrical stimulation) of acupuncture points creates a polarizing effect, increasing resistance, and thereby restoring the receptivity of cells to the heart’s electromagnetic emissions. “Lowest resistance” in physics refers to tissue with the lowest ability to receive electromagnetic signals. Electromagnetic reception depends on the electrical resistance of tissue: the greater the resistance, the better the reception (or ability of tissues to retain the electromagnetic signal). Acupuncture needling or the electrical stimulation of an acupuncture point leads to an increase in local electrical resistance, and thus the increased capacity of tissue to receive and respond to the heart’s electromagnetic emissions.
Acupuncture restores tissue organization, cell polarity and cellular function, which enables immune cells to recognize and eliminate cancer cells.
It is well known that acupuncture enhances endogenous anticancer immune function with implications for molecular–level carcinogenic control. Acupuncture improves the function and level of NK cells, activating NK cell receptor expression.50,58,59 One acupuncture point can be pivotal, though treatment protocols involving synergistic points together will produce better results. This action is realized via the activation of Beta–endorphins, especially in the lateral hypothalamus center (51, 60, 62).
It is interesting that the lateral hypothalamus area, the area of immune control activated by acupuncture, is also related to the HACER heart responses, as it has been shown that the heart’s electromagnetic field enhances the activity of this same area.60,61, leading to the activation of the biochemical immunomodulating path. Since the immunomodulating action of acupuncture stimulation is realized through the release of Beta–endorphins in the same area of the brain, it is conceivable that there is a connection between these two immunomodulation mechanisms. Indeed, it is possible that the needling of acupuncture points, which amplifies the receptivity of immune cells to the heart’s electromagnetic emissions, might activate those immune cells’ anticancer activity.61–67
In our small–scale preclinical tests, we demonstrated that it is possible to record the electromagnetic activity of the SHU acupuncture points, similar to the way current medical science records heart and brain activity using the EKG and EEG. Electromagnetic activity was detected and recorded exclusively in SHU acupuncture points, and not in inactive neighboring tissues. The recordings were different for different SHU points. Moreover, the shapes of waves recorded at SHU points corresponding to healthy internal organs were different from the waves recorded when a pathological condition was present in a corresponding internal organ.
These results suggest that the electrogram of SHU points might be employed for determining the functional status of internal organs corresponding to specific SHU points in the same way that EKG and EEG testing is used to measure heart and brain function.
In addition, our previous results point to the possibility that acupuncture points respond to the heart’s electromagnetic emissions. From those previous results, we might extrapolate that the heart’s electromagnetic emissions are distributed, at least partially, via the network of acupuncture points and meridians.
According to ancient acupuncture texts (SW Ch 8, 46), “heart radiation” plays a major role in controlling the body’s cells. This might be explained as the governing action of the heart’s electromagnetic field on the state of polarization and activity of all cells’ receptors. Through the network of acupuncture meridians, the “Emperor”, or “Invisible Heart”, regulates electromagnetic activity throughout the body, including the activity of all cells, activating complementary mechanisms of functioning, thereby accessing the body’s enormous potential for healing. This potential exists because, in addition to the body’s well–known primary physiological mechanisms, there are also complementary physiological mechanisms which remain inactive under normal conditions. For example, our bodies typically employ only 10% of available neurons, only 20% of available blood vessels, and only 10% of usable DNA. The remainder stays in reserve. Acupuncture has been shown to activate these reserves.
For instance, in experiments in rats, when scientists ligatured the coronary artery – the main source of heart irrigation, the loss of which blood supply leads to myocardial infarction – applying acupuncture to a single point (Pericardium 6) completely protected the rats from developing heart ischemia. The effect of this heart–protective mechanism of acupuncture was due to the activation of the complementary or reserve pathway for irrigation: the stimulation of this acupuncture point activated neighboring arteries – which already existed, but in an inactive state – and these arteries irrigated the heart in place of the coronary artery.
This example demonstrates acupuncture’s potential to access and activate the body’s “reserve” mechanisms. Interestingly the first point in the heart acupuncture meridian, Ht1, is called “Ultimate Spring”, which in Chinese philosophy means “endless possibility”. It seems that the ancient chroniclers of acupuncture left modern medicine a tantalizing clue to the amazing potential of acupuncture to open up the body’s innate complementary healing mechanisms.
We strongly believe in the existence of a sovereign system of regulation and regeneration of bodily tissues based on electromagnetic signals generated by the heart and distributed via the network of acupuncture meridians. This system controls the organization and maintenance of tissue structures, as well as the state of polarity, and functional activity of all cells, including immune cells. A disturbance in this system that leads to disorganization of cells or tissues in an organ might explain a pathological condition in that organ, since disorganization can lead to disease. Tissue or cell disorganization might occur as a result of faulty genetics or due to an acquired condition that causes tissues to lose their ability to receive and respond appropriately to the heart’s electromagnetic instructions. This can occur when local electrical resistance decreases and tissues become unable to receive and/or retain the heart’s electromagnetic emissions.
This explains why acupuncture points are known to have lower electrical resistance in the presence of a pathological condition: the lower the electrical resistance, the weaker the response of tissues in a corresponding organ to the electromagnetic communication, which consequently predisposes the organ to disease.
The propagation of the heart’s electromagnetic signals through the network of acupuncture meridians would explain the physiological action of acupuncture. The needling of an acupuncture point leads to increased local electrical resistance in the target tissues, resulting in increased receptivity and responsiveness to the heart’s electromagnetic control, and restoring organization of tissues through the restored polarity and activity of cell receptors. In addition, acupuncture amplifies the intensity of electromagnetic emissions, improving their ability to regulate tissue organization. This explains why the strong manipulation of acupuncture needles, including vibration and shaking, intensifies the efficacy of acupuncture: such manipulations increase local electrical resistance and thus receptivity to the heart’s electromagnetic signals.
In addition, several studies have shown that acupuncture enhances the flow of stem cells through acupuncture meridians toward a target organ. This increased supply of stem cells replaces the destroyed cells and thus aids in regeneration of tissues. In our opinion, electromagnetic signals generated by the heart regulate the flow of these cells through the acupuncture meridian, and thus control the body’s innate potential for regeneration.
Our preliminary research offers additional proof for the existence of a sovereign system of control over the organization and function of bodily tissues, the foundation of which is electromagnetic communication generated by the heart, and propagated through the network of acupuncture meridians. The recognized immunomodulating effect of acupuncture is related to the restoration of tissue structure and organization, and cell polarity and function. The needling of acupuncture points activates the receptivity of target tissues and immune cells to the heart’s electromagnetic signals and thus restores their polarity and function.
There are no competing financial interests to declare.
None.
Author declares that there are no conflicts of interest.

©2017 Volf, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.