International Journal of
eISSN: 2574-9862


Research Article Special Issue Marine Biology
1Wildlife Conservation Society, 2300 Southern Boulevard, Bronx, New York, 10460, USA
2Section of Ornithology, Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, CA 90007, USA; Current address Casilla 19, Punta Arenas, Chile
3Wildlife Conservation Society, Chile
4Feather Link, 1013 Westchester Way, USA
Correspondence: David Oehler, Wildlife Conservation Society, WCS/Bronx Zoo, 2300 Southern Boulevard, Bronx, New York, 10460, USA
Received: June 27, 2018 | Published: August 1, 2018
Citation: Oehler DA, Marin M, Kusch A, et al. Foraging ranges in Southern Rockhopper penguins (Eudyptes chrysocome chrysocome) on Isla Noir, Chile. Int J Avian & Wildlife Biol. 2018;3(4):320-325. DOI: 10.15406/ijawb.2018.03.00109
The foraging range during the breeding season and Austral winter period of Southern Rockhopper Penguins, Eudyptes c. chrysocome, has been previously documented within colonies along the coast of Argentina and in the Falkland (Malvinas) Islands. We examined annual movement patterns of Southern Rockhopper Penguins to better understand the utilization of their environment in southern Chile. 25 Southern Rockhopper Penguins from Isla Noir were equipped with global location sensors that logged light-levels, water immersion (wet/dry) and sea-surface temperatures (SST) providing daily position, water immersion, and environmental conditions. Five of the penguins were relocated on Isla Noir for data retrieval. Position data were converted into maps of kernel density estimates overlaid with average SST. Study penguins had greatest densities near the colony during the breeding season and dispersed to areas ranging from near the Falkland Islands to areas between 46°S to 57°S and 114°W to 97°W. The penguins utilized areas over the continental shelf during the breeding season and ranged to areas over the ocean basin during the Austral winter period, post-breeding season and molt. Coastal areas along the outer islands were used during the transitional periods between the breeding season and winter period. These data are important in defining the conservation management of the Southern Rockhopper Penguin in relation to fisheries-associated mortality and depletion of food sources.
Keywords: activity patterns, at-sea distribution, Eudyptes chrysocome chrysocome, foraging behavior, Geolocation, tracking, southern Rockhopper penguin
The Southern Rockhopper Penguin, Eudyptes c. chrysocome, breeds on the Falkland/Malvina Islands, Isla Pingüino, and Ilsa de los Estados in the Atlantic Ocean and on Barnevelt, Terhalten, Buenaventura, Ildefonso, Noir and Deigo Ramirez islands around Cape Horn in the Pacific Ocean, South America.1‒3 The International Union for Conservation of Nature (IUCN) lists the Southern Rockhopper as Vulnerable on the Red List of Threatened Species.4 This South American population is estimated to consist of approximately 870,000 pairs of which the colonies on the outer island of Chile account for 46% of that total.5 Within this area, the 158,200 pairs on Isla Noir represent the largest concentration of Southern Rockhoppers along the Chilean coast.2,6 The IUCN notes declines in these population of 34% over the last thirty years that may be attributed to egg collection and other anthropogenic pressures have been recorded.7‒9 Hydrocarbon exploitation, interactions with fisheries, climate change, possible competition with increasing pinniped populations and newly developing aquaculture activities involving salmon are additional factors that may have or continue to place pressures on the Southern Rockhopper Penguin.4,10‒15 Mean survival rates in the Southern Rockhopper Penguins, in the Falkland Islands, were 84 to 96%.16
The distribution of penguins during the breeding season is dependent on the distribution of prey and proximity to nesting areas.17 The geographical range of penguins expands during the non-breeding season when compared to the breeding season periods.18 Rockhoppers have a high site fidelity to their natal colonies and inter-annual fidelity in breeding of 59%.19,20 Post-breeding dispersal and foraging patterns during the breeding season for Southern Rockhopper Penguin has been documented for colonies within Staten Island, Argentina, and the Falkland/Malvinas Islands,21‒24 but no studies have been conducted in colonies along the coast of Chile. A lesser amount of data is available involving the arrival and dispersal patterns of Southern Rockhopper Penguins breeding on the outer island along the Austral Chilean coast than is available from the Eastern coast of South America. Even less is known about their migration patterns, once they depart from the breeding colonies. The winter range is presumably pelagic with winter observations on the coastline demonstrating that a number of Southern Rockhopper Penguins have traveled along the Humboldt Current as far north as Punta Talca, Cachagua Island and Santo Domingo (30° to 33°S).6 The high biological productivity associated with Chile’s coast supports major fisheries and seabird colonies. The impact of fishing activities, both industrial and artisanal, including mortality events involving Southern Rockhopper and longline fisheries, and other environmental factors may pose a threat to the seabirds in the Chilean waters.25 Since the mid-1980’s increased salmon farming along the coast had resulted in increased nitrogen and phosphate loads that alter the plankton taxa within those areas and intraspecific pathogen transmission.13,14
Using wing bands on penguins remains contentious due to possible adverse long-term effects on the survival, reproduction, and behavior of the marked.26‒30 In additional studies, properly fitted stainless-steel bands were shown to have little impact on the long-term survival or had varied effects when examined over several seasons.26,31 Recommendations consist of alternative marking techniques including the use of PIT tags and automatic readers as penguins enter or exit the breeding colony and use of GLS tags mounted on leg.27‒30,32 Suitable conservation research practices should reduce the number of animals used in an experiment while remaining statistically relevant, refining procedures to minimize pain and distress in the experimental subjects and provide for the well-being based on their behavioral needs. Furthermore, these practices should be minimally invasive, reduce animal welfare impacts and determine the best attachment methods minimizing those adverse effects, including temporal elements.33
In this paper, we compare foraging ranges of Southern Rockhopper Penguins from Isla Noir and how those ranges vary between the breeding season, including the period of molt, and the post-breeding period. The goal of the study was to document the habitat preferences during these two periods. The use of kernel density estimates allows for the mean modeling density surface to illustrate these findings. Monitoring populations and annual migrations of penguins, such as the Southern Rockhopper Penguin, may provide a better understanding of the pressures on these populations and provide policymakers with information on the performance of their conservation and resource management measures and data to increase the protection of the vital zones.
As a precursor to this study, we conducted ex-situ and in-situ trials of wing band and leg band attachments of the tracking devices. Several studies reported key long-term negative effects utilizing wing bands in King Penguins, Aptenodytes patagonicus.27,30 To reduce these negative effects, during a short-term study, we tested novel wing band designs on captive Rockhopper Penguins and minimized the size and weight of the tracking device. Initial field trials employed both wing and leg bands to determine if the short-term effects negatively impacted nest attendance or caused abrasion or disruption in waterproofing.
Two captive Rockhopper Penguins were fitted with the wing-bands and attached GLS tags at the Cincinnati Zoo & Botanical Garden in June 2008. For this application, we attached “dummy” GLS units and released the birds back into the sub-Antarctic seabird display. Daily observations involved normal notations of behavior and examination of the banding site. These bands and units were removed after a six-month deployment.
We conducted a field trial of the GLS tags on 13 November 2009, in the Southern Rockhopper Penguin colony on Isla Noir, Chile to determine the efficacy of wing-band versus leg-band attachment. A total of 18 adult Southern Rockhopper Penguins were equipped with non-transmitting GLS tags; nine wing-bands and nine leg-band attached GLS. Retrieval of the units was completed on 11 November 2010. Programming of the units involved the collection of locational data points between 13 November 2009 and 1 March 2010.
Study Area and study period
Field activities were conducted on 14 December 2012 and 12-15 and 25 November 2013, in the Southern Rockhopper Penguin colony on Isla Noir, Chile (54.46°S, 72.97°W). All scientific research permits were obtained to visit and conduct this scientific investigation through the Chilean Government.
Geolocational tracking
A total of 25, approximately 50% male and 50% female, adult Southern Rockhopper Penguins, were equipped with non-transmitting geolocational archival (GLS) tags (Lotek Wireless Inc., Newmarket, Ontario, Canada) to observe foraging and migration patterns. Five penguins were relocated and the GLS tags retrieved on 25 November 2013 and data were downloaded successfully from those tags. The GLS tags were attached to stainless steel wing bands shaped to minimize hydrodynamic drag. We selected GLS tags because of the tags’ ability to withstand a marine environment and their relatively small size (8x32mm, ˂5 g). The tags recorded data every two minutes logging light-levels, water immersion (wet/dry), and sea-surface temperature (SST). Deployment and subsequent retrieval of the GLS tags were timed to coincide peak colony attendance; penguins were tagged in December 2012 and recovered during November 2013. Southern Rockhopper Penguins remain ashore during portions of the nesting season and during their molt in the Austral autumn, staying at sea for several weeks in the Austral spring as they establish territories prior to the breeding season.34 The commencement and end of the winter period were determined via immersion records of the GLS tags and confirmed through the use of camera trap images recorded daily during the study period (Oehler unpublished data). Mean daily sea-surface temperatures were recorded throughout the entire GLS tag deployment period.
Estimates of daily location were computed from recovery light-level data by Lotek from standard celestial algorithms. Any locations derived from these data with an error ≥120km were noted during processing and subsequently excluded. Light-level data before the end of the period affected by the spring (18-22 March 2013) or autumn (20-24 September 2018) equinox were discarded based on unreliable estimations based on light duration. Foraging locations were overlaid with monthly average sea surface temperature to assist in verification of locational data.
Maximum distance from the colony was calculated as the straight line from the colony to the most distant location received each day throughout the year. Daily maximum distance from the colony was compared among individual penguins.
At sea location data were imported to ArcMap 10.3.1 for Desktop and plotted, then overlaid with monthly average SST data from NOAA’s Optimum Interpolation Sea Surface Temperature V2 dataset (http://www.esrl.noaa.gov/psd/data/gridded/data.noaa.oisst.v2.html). Kernel analysis results were divided into seasonal categories involving the breeding season and winter ranges. Probability polygons of foraging density were produced showing 50% and 95% isopleths.
Ex-situ trials: Daily observations involving the captive Rockhopper Penguins, maintained at the Cincinnati Zoo & Botanical Garden, did not denote any device-induced behaviors. Feather wear at the terminal end of the wing-band was noted.
In-situ trials: Results of the first field trial of the GLS units demonstrated minor feather wear at the terminal end of the wing-bands and minor dermal abrasions on the legs involving the leg-band attachment. 15 (8 wing-band attachment and 7 leg-band attachment) units were retrieved representing 83% of the units deployed correlating with normal rates of return to the colony by adult Southern Rockhopper Penguins. Data from these units were downloaded and data points were filtered due to eliminating excessive error rates of ≥120. Wing-band based GLS units successfully recorded daily location data an average of 36.6 days over the trial period. Leg-band units successfully recoded daily locational data an average of 19.1 days during the same period.
Upon completion of the 2012/2103 season and the retrieval of the tracking units, no obvious physical effects of the device attachment to the wings and/or body condition were noted.
Filtering due to excessive error rates of ≥120km removed 66% of all the GLS location results. Clear differences in foraging ranges were apparent between the breeding season including the period of molt and post-breeding winter dispersal period (See Figures 1–3). Median maximum distances from the Isla Noir colony reached by foraging birds were 1118km (SD=697) post-breeding/molt dispersal.
During the period involving nesting and molt (October through March) the adult penguins foraged in the area southeast of Isla Noir. The extent of the foraging range is between 48°S to 59°S and 80°W to 70°W. During February and March when the penguins remain on Isla Noir to undergo a complete molt, they forage in the area between 50°S to 64°S and 84°W to 69°W. During October, 21% of the known locations were detected along the near coastal areas with ocean depths of less than 3000m.
The results of the kernel analyses during the breeding season were overlaid with average SST and are illustrated in Figures 1&3. Densities were greatest near the colony and within the West Wind Drift Current and the flow into the Cape Horn Current over the Mornington Abyssal Plain, but also include trips into the Straights of Magellan. Foraging areas within the 50% isopleth covered 11,713km² (December 2012–March 2013) and 17,107km² (October–November 2013). Within the 95% isopleth ranges covered 124,486km² (December 2012–March 2013) and 108,103km² (October–November 2013).
Abundance indices, within the colony, were determined based on location data and wet/dry data to outline the phenology. The last penguins departed the colony by 28 March after completion of their annual molt. The first of the adult Southern Rockhopper Penguins returned to the colony beginning on 3 October with peak attendance recorded on 23 and 24 October. The abundance of penguins, based strictly on the indices, was reduced by 50% on 2 November when compared to peak attendance.
Winter dispersal correlates with the departure of the penguins in April and their return to Isla Noir in September. The results of the kernel analyses during the winter period season were overlaid with average SST and are illustrated in Figure 2. During the April – September period the foraging areas within the 50% isopleth covered 465,501km² and the 95% isopleth covered 1,929,341km². In April, the penguins dispersed to diverging sites. Three female penguins foraged in an area between 44°S to 57°S and 91°W to 79°W. One individual male moved farther west and ranged between 46°S to 57°S and 114°W to 97°W. The remaining male penguin traveled around Cape Horn and the foraging area encompassed the area between 59°S to 57°S and 84°W to 73°W, including the region near the Falkland Islands. In July the penguins ranged between 46°S to 57°S and 58°W to 113°W. This range involves the Cape Horn Current as well as the Malvinas Current which one individual bird utilized.
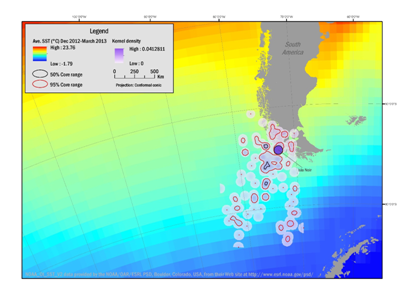
Figure 1 Range of Southern Rockhopper Penguin during the breeding season and pre-molt, based on kernel range analysis December 2012 through March 2013 from the breeding site (line to Isla Noir) and foraging areas of three females and two males. Density contours reflect 50% (black boundaries) and 95% (red boundaries) correlating with average sea-surface temperatures (SST).
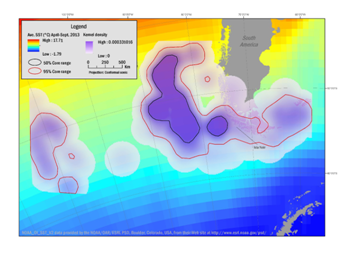
Figure 2 Winter dispersal range of Southern Rockhopper Penguin based on kernel range analysis April through September 2013 from the breeding site (line to Isla Noir) of three females and two males. Female penguins ranged in an area between 44°S to 57°S and 91°W to 79°W with one male migrating west between 46°S to 57°S and 114°W to 97°W and the second male traveling around Cape Horn to an area between 59°S to 57°S and 84°W to 73°W. Density contours reflect 50% (black boundaries) and 95% (red boundaries) correlating with average sea-surface temperatures (SST).
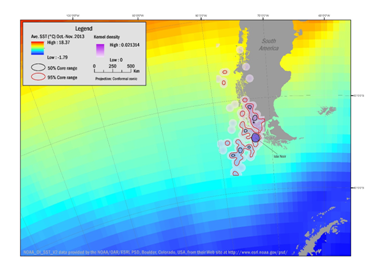
Figure 3 Range of Southern Rockhopper Penguin based on kernel range analysis October through November 2013 highlighting the transitional route between the winter dispersal range to the breeding site (line to Isla Noir) of three female and two male penguins based on kernel range analysis. Density contours reflect 50% (black boundaries) and 95% (red boundaries) correlating with average sea-surface temperatures (SST).
Excluding data from January, the mean SST temperatures recorded by the GLS range from 4.16°C in May to 5.33°C in April. The mean SST temperature for January was 7.06°C.
This study represents a short-term investigation to determine the annual migration of Southern Rockhopper Penguins. Due to poor weather conditions, retrieval of the GLS tags was limited, although previous trials involved higher rates of retrieval at 83%, which is within normal nest fidelity and survival return rates. It is possible that penguins taxed by GLS tags may require longer foraging periods to maintain body condition when compared to penguins that do not carry such instruments.35,36 Variances in foraging durations could not be determined within the context of this study. We did conclude that the body condition based on plumage condition of the Southern Rockhopper Penguins that carried the GLS tags for one year was not significantly altered during the study period. The studies conducted were restricted to one-year periods to reduce the stresses involved on the individual penguins. Alternative tracking platforms were eliminated from consideration due to poor data collection in terms of leg mounted GLS units, increased platform size with GPS or satellite tracking units and unsuitability in tracking in a pelagic environment, such as PIT tag marking.
These data describe the annual foraging patterns and distribution of Southern Rockhopper Penguins from Isla Noir, for the first time. The post-breeding winter foraging areas diverged from those observed during the breeding season and period of molt and were larger than those detected during the previous surveys from land or sea-based platforms.2 Habitat preferences deviated during the breeding season and period of molt and the Austral winter period. Further lack of consistency between individual penguin during the winter period was observed with birds ranging from areas along the continental shelf to ocean basin, contradicting predictions of like habitat dependency. During the reproductive and molt period, the birds are concentrated within the Cape Horn Current, near Isla Noir, which is characterized by a large upwelling system and productive marine ecosystem. During this period the Southern Rockhopper Penguins forage for crustaceans, fish, and cephalopods that are associated with these steep gradients in SST and salinity.24,37 During the non-reproductive period, we expected a more northerly migration based on previous field observations, which occurred, although some individual birds moved southeast and west increasing the extent of that range and overlapping the foraging ranges of populations that breed on the Falkland and Staten Islands. Prey aggregation during this period could not be determined, based on the current set of criteria established by the GLS data provided without further direct observation of the remote locations or individual penguins. By utilizing the location, SST data, along with the correlating wet/dry information of the GLS tags were are able to confirm the relative location of the penguins, on a seasonal basis.
The use of GLS tags allowed for continued monitoring of the Southern Rockhopper Penguins once they departed from Isla Noir for up to one year. The removal of location data involving a mean error of ≥120km did not affect the identification of foraging areas during the breeding season and Austral winter. Although transition between these periods is somewhat lacking, the use of kernel density estimates has provided delineation of boundaries around high densities of the Southern Rockhopper Penguins within the coastal areas of Chile. The distances traveled during these periods almost certainly accounted for the consistent errors in logging daily locations. The GLS tags provided sufficient data to identify foraging ranges in these pelagic birds and highlight the need to further study annual movements in addition to the foraging range during the nesting season due to possible inter-annual variability influenced by the variability in oceanographic systems.38
Through the Convention on Biological Diversity, governments have pledged to safeguard 10% of the world’s oceans areas, establishing Marine Protected Areas (MPAs), although the utilization of seabird data is only gradually being incorporated into these processes.39 In Chile, the main legal tools that exist for the implementation of MPAs take the form of Natural Sanctuaries, National Monuments, Marine Parks, Marine Reserves and Multiple-use MPAs.40,41 Despite funding constraints, the number of MPAs has increased significantly in the past years, although the expansion of these areas has been recommended to increase protection, particularly within the extensive fjord regions and Exclusive Economic Zones where there are no protected areas.42‒44 This study provides data that may be utilized to identify areas of interaction and potential conflict with commercial fisheries resulting known mortality events involving longline fisheries and potential injury or death from gillnets and purse seines in penguins.11 Merging these data with ongoing goals of the Multiple-use MPAs will allow for effective conservation management of birds such as the Southern Rockhopper Penguins in which fisheries-associated mortality or depletion of food sources may result in population declines.
We dedicate this work to W. Roger Fry (1940–2017). This work was supported by Feather Link, Incorporated and by grants-in-aid for scientific research from local and international foundations. We thank the Angel Fund, African Safari Wildlife Park and the Wildlife Conservation Society for their support. J. Alan Clark and Bonnie Raphael provided helpful suggestions and insights for the final manuscript. Permits were provided by the Departamento de Protecci\n de Recursos Naturales Renovables Subdepartamento de Vida Silvestre. We also are grateful to all the people that helped with field work including Hugo Cardenas, W. Roger Fry, Rodrigo Gonzalez, Alberto Gutierrez, Alejandro Kusch, Kimberly Lenhardt, Joanne Rapley, Susan Schmid, and Leonard Weakley. A special thank you to Nicolas Pivcevic, Aerovías DAP, for the helicopter transport to Isla Noir, when weather conditions prohibited water transports.
The author declares that there is none of the conflicts.

©2018 Oehler, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.