International Journal of
eISSN: 2574-9862


Research Article Volume 1 Issue 1
Wisconsin Veterinary Referral Center (WVRC), USA
Correspondence: Arboleda CV, Wisconsin Veterinary Referral Center (WVRC), 7718W Green Tree Road, Milwaukee, WI 53223, 300 Seward Street, Ripon, Wisconsin, USA
Received: January 31, 2015 | Published: January 4, 2016
Citation: Arboleda CV, Khan MZ. Testosterone and heterospecific aggression in the adult eastern bluebird (sialia sialis) during the nestling period. Int J Avian & Wildlife Biol. 2016;1(1):5-11. DOI: 10.15406/ijawb.2016.01.00002
Conspecific competition is assumed to exert stronger selection than heterospecific competition on the aggressive behavior and its underlying physiology in birds. We tested the hypothesis that hormone-behavior interaction between testosterone and aggression may also be shaped by severe heterospecific competition among secondary cavity nesters. Adult Eastern Bluebirds (n=34) were exposed to three challenge conditions that simulated territorial intrusions (STI) by heterospecific (House Sparrow) and conspecific using live and stuffed decoys with their respective playback vocalizations. Eastern Blue birds were responsive to simulated territorial intrusions (STIs) by Conspecific and heterospecific late in the nesting cycle. The aggressive response elicited from a heterospecific STI was muted compared to honspecific STI. Overall, highly aggressive individuals had slightly greater mean testosterone concentrations than individuals with low aggression scores. Baseline T and aggression were positively correlated for all STI conditions, but the relationship was strongest for the live heterospecific decoy. Furthermore, the live heterospecific decoy elicited a stronger aggressive response than the stuffed decoy suggesting that other cues produced by the living bird, in addition to the auditory and visual cues provided influenced individual responses to a challenger.
We conclude:
Keywords: breeding season, interspecific aggression, intraspecific aggression, testosterone, simulated territorial intrusion
During the breeding season, birds establish territories and aggressively defend them against intruders. In general, conspecific intruders elicit greater behavioral responses than heterospecific because heterospecifics are not usually competing for the same resources. Interference competition, through aggressive interactions between species, has the potential to affect community structure.1 In male birds the steroid hormone testosterone, mediates territorial aggression.2 Testosterone and aggression has been studied relative to seasonal cycles3 and population density Beletsky et al.4 Species differences in testosterone and aggression may influence their dispersal ability5 and geographic distributions.6 Aggression towards conspecifics and heterospecifics may be mediated by the same physiological pathways and we test this hypothesis here.
Nest box trails, a popular management tool, provide an opportunity to examine heterospecific competition because of relatively small distances between cavities and placement of boxes in suburban environments which harbor nest box competitors like House Sparrows (Passer domesticus) and European Starlings (Sturnus vulgaris). Among secondary cavity nesting birds, interspecific aggression is more common because of limited cavity availability. For example, Collared Flycatchers (Fidecula Albicollis)are most aggressive toward Great Tits (Parus major), another cavity nester, and least aggressive toward Dunnocks (Prunella modularis), an open-cup nester.7
The challenge hypothesis states that circulating testosterone rises above baseline concentrations in response to competition for mates and territory. A rise in testosterone in response to social challenges strengthens subsequent sexual displays and prolongs the duration of aggressive behaviors. 8-10Tests of the challenge hypothesis focus on behavioral and physiological responses to conspecific intrusion11,12 because conspecific competition is assumed to be stronger selective factor than heterospecific competition. This assumption is under scrutiny and should be tested.1 We tested the hypothesis that testosterone and aggression levels may correlate with heterospecific competition as well.
We examined the relationship between baseline circulating testosterone and the expression of aggressive behavior of adult eastern bluebirds to different competitor challenges during the late nestling period. Eastern Bluebirds, Sialia sialis, are obligate secondary cavity nesters that compete with other cavity nesting species. We addressed two questions in this study. First, are Eastern Bluebirds responsive to simulated territorial intrusions (STIs) by conspecifics and heterospecifics late in the nesting cycle? Even after establishment of territories they remain at risk of intrusions by House Sparrows13,10 and male bluebirds.14,15 Both types of intrusion may compromise an individual’s reproductive success, conspecifics via cuckoldry14 and sparrows by direct mortality to nestlings.13 Furthermore, we expected males to react more aggressively to conspecific challenges because in a reproductive perspective, males are the only individuals for whom a conspecific intrusion is a greater disadvantage.
Second we asked if baseline testosterone concentrations and aggressive behavior covary within individuals. We predict individuals with high baseline testosterone concentrations will exhibit more aggression than males with lower testosterone concentrations. We used baseline testosterone because it likely indicates the long-term social environment of the individual. Males exposed to more territorial intrusions exhibit higher baseline T than males in less competitive environments.16
Monitoring
The study area consisted of nest boxes on the grounds of Ripon College, backyards and municipal parks of Ripon, Wisconsin (43˚50´40˝N 88˚50´20˝W). Nest boxes were monitored weekly from April-August in 2009 to determine occupancy by bluebirds, nesting phase and nest success. Once a bluebird nest was identified, boxes were checked twice a week during the incubation period (12-14 days). Daily monitoring of bluebird nests started two days before hatching, and continued until day 13 to avoid premature fledging.17
Capture and blood sampling
The relationship between aggression and circulating testosterone concentration in male and female eastern bluebirds was examined by correlating behavioral responses to simulated territorial intrusions (STIs) to hormone concentrations measured in blood plasma. Adult birds (N=34) were captured with remote control nest- box traps when nestlings were ten days old. We used a mist net and a conspecific decoy with playback vocalizations as a method of capture when the remote trap was not effective. Because capture may affect hormone concentrations in blood8 the method of capture and the time between capture and blood collection were included in subsequent statistical analyses. Blood samples were collected from adult birds within 6.53 ± 0.34min (mean ±SD) after capture, well within the ten minute window before the stress of capture causes testosterone to decline.18 For blood collection, we punctured the brachial vein with a 26 gauge needle and drew approximately 240µl of blood using heparinized microhematocrit capillary tubes.8 Blood samples were placed into a cooler in the field, and transported to the lab within four hours to be stored at -80˚C. During capture we measured each bird’s mass (g), right tarsus length (mm), and right wing chord length (mm) to reflect an individual’s quality. Unbanded individuals were banded with a unique color combination of bands (Master banding permit #23473) and a USFWS numbered aluminum band.
Behavioral observation
Most observations started a day after capture of the box (unless observations occurred during capture), when nestlings were 11 days old, and were completed on day 13 (three days after capture) so that simulated intrusions did not influence the measurements of circulating testosterone.
We modified methods from Duckworth10 & Wingfield et al.19 We simulated a conspecific intrusion by placing a prepared skin of a male Eastern Bluebird (conspecific decoy=CD) on top of the nest box while playing a recording of the bluebird songBecause bluebirds have aggressive encounters with House Sparrows even after establishment of territory13 we simulated a heterospecific intrusion by presenting a House Sparrow while playing a recording of its song. Heterospecific intrusions were modeled in two ways, with a prepared skin (heterospecific decoy=HD) or with a living bird in a cage (heterospecific caged =HC). Each nest box was exposed to all three conditions (CD, HD, and HC) in a randomly assigned order, with each observation lasting approximately five minutes (4.40±1.66 min).
Some observations were terminated earlier because of an unexpected equipment failure, or because the decoy was damaged by attacks. We recorded behaviors using a tape recorder, and a Flip Video mini-HD camera placed approximately 15m from the box on a tripod. To quantify aggression we analyzed video playbacks and measured the latency of first attack on the decoys in addition to the number of attacks, hovering, and fly-overs. All behaviors were analyzed as frequency per minute to control for the different observation times during each STI. Vocalizations were also recorded, but genders were combined because we could not distinguish the vocalizations of males from females. We did not analyze vocalizations during trials with conspecific decoys because we could not distinguish the calls made on the recording from the calls made by the defending birds.
Aggressive behavior was scored on a scale of 1-6 as described by Duckworth10 one indicating a slight response and six a strong response (Table 1). We standardized our behavioral data by dividing the frequency by the time observed and multiplying by two to be consistent with Duckworth’s criteria of 2 minute observations.
Score |
Behavior |
Qualitative Description |
|
Number of Times Flying or Hovering |
Number of Attacks |
||
1 |
0 |
0 |
No aggressive behaviors |
2 |
1-5 |
0 |
Minimal response, moderately aggressive behaviors |
3 |
>5 |
0 |
Moderate response, moderately aggressive behaviors |
4 |
-- |
1-5 |
Moderate response, highly aggressive behaviors |
5 |
-- |
6-9 |
High response, highly aggressive behaviors |
6 |
-- |
>9 |
Very high response, many highly aggressive behaviors |
Table 1 Criteria for scoring aggressive behavior of eastern bluebirds based on.2
Hormone analysis
Blood samples were centrifuged in hematocrit capillary tubes at 3460xG for five minutes in order to separate red blood cells from plasma, and the plasma was transferred into 0.5 ml microcentrifuge tubes using a Hamilton syringe. Final samples were stored at -80˚C until laboratory analysis.
We used methods described by O’Fegan20 to validate the Salivary Testosterone Enzyme Immunoassay (EIA) kit (Salimetrics). The EIA kit is capable of measuring testosterone in small-volume avian plasma,21 and is sensitive enough to detect biological changes in the gonadal activities of small birds.22 The slopes of serial dilutions were parallel to the standard curve for male plasma (t=-0.25, p=0.81) and female plasma (t=-0.11, p=0.91) indicating that bluebird plasma does not interfere with the ability of the anti-testosterone antibody of the Salimetrics kit to bind to testosterone in avian blood samples.23 Intra-assay variation for high controls was 1.63%±2.00 and12.69% ±6.09 for low controls. Inter-assay variation was 7.37% and 7.60% for high and low controls respectively. Male samples were diluted to 1:4 plasma/ assay diluents and female plasma was not diluted due to the expected low concentrations of testosterone. Plasma from individuals with very low testosterone concentrations was spiked using 24pg/ml standard and unknown concentrations were then back calculated.
Statistical analysis
Statistical analyses were performed using SPSS 19.0 (IBM, Chicago, IL USA) and.24 For this study we performed all behavioral analysis using parametric statistics and corrected for small sample sizes using the Bonferroni method. A t-test was carried out to compare accuracy of measure by voice recording and a video camera. There was no statistical difference between the data taken by either medium (t50=0.40; P=0.69), therefore I assumed that the same conclusions would be reached by analyzing either one. I chose to analyze fly-over data collected by voice recording because the field of view of the video camera was too narrow. Attacks, vocalizations and hovers were analyzed using video recordings.25
The effect of conspecific or heterospecific intruders on aggressive behavior was examined using a Multivariate Analysis of Variance (MANOVA), where rates of behaviors were compared among the treatment conditions and between males and females. Testosterone concentrations in males and females were compared using a t-test. The relationships between testosterone and aggression scores were analyzed with linear regression. All results are presented as mean ± standard deviation unless noted otherwise.
Simulated territorial intrusions
The simulated territorial intrusions elicited responses from the bluebirds (1.08±0.31 min, N=23). Males (0.72±1.14 min, N=18) responded more quickly than females (1.45±2.12 min, N=7). Both adults reacted differently to the simulated intrusions of heterospecific and conspecific competitors (F2=10.61, P< 0.001). The amplitude of their response was greater if the decoy was a living bird compared to a stuffed skin. Both sexes attacked, hovered and flew-over the living House Sparrow more often than the stuffed decoy (Figure 1). The total number of vocalizations produced by both males and females was significantly greater toward the living House Sparrow (Figure 2, one-way ANOVA, F1=0.88, P<0.006). All other comparisons were restricted to simulated intrusions with stuffed decoys because we were more interested in the comparing aggressive responses of bluebirds toward conspecifics and heterospecific intruders.


Aggression
Males tended to be more aggressive than females toward any decoy when attacking (Figure 1; F1=16.29, P=0.00), or hovering (F1=2.85, P= 0.10) but not when flying over the decoy (F1=1.21, P= 0.730). Overall, the resident pair acted more aggressively toward the conspecific decoy than the heterospecific decoy during attacks (F1= 4.87, P=0.03) and hovers (F1=9.81, P=0.003) but not during fly-overs (F1=1.26, P= 0.27).
Testosterone and aggression
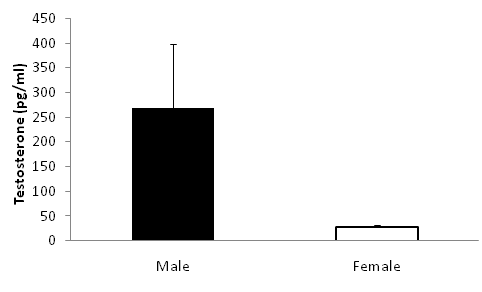
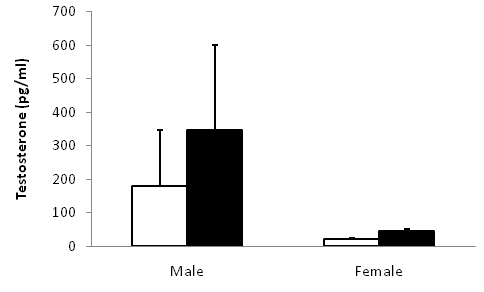
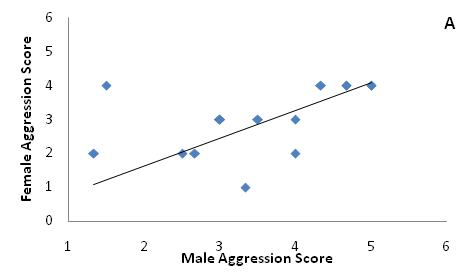
Males exhibited marginally higher testosterone concentrations than females (Figure 3, t=1.88, p=0.05). On average, individuals that exhibited high aggression did not differ in circulating testosterone than individuals that exhibited low aggression among males (Figure 4, one-way ANOVA, F1=0.16, P=0.71) or females (F1=2.34, P=0.16). Females paired to males had weakly correlated aggression scores (Figure 5A, F1=4.03, P=0.07) and testosterone concentrations (Figure 5B).
We explored the relationships between aggression scores and baseline testosterone for male bluebirds in each of the three STIs. Every STI exhibited a positive correlation between baseline testosterone concentration and individual aggression scores (Figure 6A-6C) but the relationship was insignificant for responses to the conspecific decoy (F1=0.57, P=0.48), and the heterospecific decoy (F1= 0.80, P=0.40). Interestingly, the positive correlation between testosterone and aggression was marginally significant for the male bluebird’s response to the heterospecific caged decoy (F1=5.03, P=0.06).
Are bluebirds responsive to territorial intrusion late in the nesting cycle?
Eastern bluebirds compete fiercely for limited cavity space against other cavity nesters even after establishment of territories. Based on the challenge hypothesis we predicted that Eastern Bluebird aggression would be maintained late in the nesting cycle because of their vulnerability to intrusions by both conspecifics and heterospecifics. In this study, STIs by conspecific and heterospecifics elicited aggressive responses by males and females, indicating that eastern bluebirds perceive conspecific and heterospecific intruders as a threat.
Among males, the level of response toward the heterospecific decoy was lower than toward the conspecific decoy. Perhaps the risk of nest failure from House Sparrow cavity usurpationis less than the risk of losing paternity to a conspecific male. Eastern Bluebirds in the Midwestern US attempt second broods,26 so males may still need to guard against sexual competitors late in the nesting cycle of first broods. To determine if Eastern Bluebirds are responding to risk probabilities, we suggest examining aggressive responses under conditions of different conspecific and heterospecific density and intruders with different risks. For example, one should compare the aggressive responses of Eastern Bluebirds to Black-capped Chickadees who pose little risk of nest box usurpation to that of House Wrens (Troglodytes troglodytes) which often destroy Eastern Bluebird nests. Lehtonen demonstrated that cichlid fish modulated their aggressive responses depending on the risk (breeding or non-breeding colors) posed by a heterospecific intruder.
We measured Eastern Bluebirds aggression using three different metrics, direct attacks, hovering, and flyovers. Compared to males, females were less likely to attack and more likely to flyover conspecific decoy. These different responses could be explained by the different reproductive strategies of each sex. Eastern Bluebirds are known to double brood and occasionally change mates during a given breeding season.27 Male Eastern Bluebirds mate-guard and drive away sexual competitors15 who explain the attack behavior observed in this study. Female flyover behavior may be interpreted as her assessment of a potential new sexual partner.
Do baseline T and aggressive behavior covary among individuals?
Males exhibited marginally higher circulating testosterone than females which is not surprising given that blood sampling occurred late in the nesting cycle, suggesting that testosterone levels in males are lower than at the beginning of the nesting period. In socially monogamous double-brooded species, circulating levels of testosterone reach a peak during the egg-laying phase, and remain at lower levels during the parental phase and into the second sexual phase.28 Male Eastern Bluebirds provide parental care during the nestling and fledgling phases.29 In many species parental behavior is depressed if testosterone is experimentally elevated revealing a trade-off between current and future reproductive success.30 That we observed similar male and female testosterone concentrations in this study likely represents this trade-off in Eastern Bluebirds.
Highly aggressive individuals had marginally greater mean baseline testosterone concentrations than individuals with low aggression scores, but the differences were not statistically significant. We assumed that individual differences in baseline testosterone could be attributed to differences in social context and not seasonal variation because each bird was sampled at a standardized time in the nestling cycle, when nestlings were 10 days old. However, circulating levels of androgens may only correlate with aggression during periods of instability. We assumed instability in this study because nest losses to House Sparrow usurpation occur throughout the breeding season (pers. obs) but this assumption was not tested. Future studies should test this assumption by quantifying heterospecific density in the study area. We also assumed that social interactions had an additive effect on baseline testosterone such that males with higher baseline T were exposed to greater social instability. Recent work on Great Tits and Stonechats indicate that this assumption may be naïve.31 Future studies should measure both seasonal testosterone patterns and the changes in testosterone in response to STIs.
Other mechanisms may contribute to aggressive behaviors during the parental phase as well, when testosterone needs to be maintained at lower levels. Testosterone remains at higher than basal levels throughout the breeding season for many species,8 but it seems to contribute little to aggression observed during the non-breeding season.32 The variability of aggressive responses among individuals and the lack of apparent relationship with their respective testosterone levels could be attributed to individual sensitivity to circulating testosterone,30 or other hormones such as estrogen may mediate aggression when testosterone is low.33
Our data show a weak correlation between aggression scores and testosterone concentrations of breeding pairs. Recent studies have demonstrated that the testosterone cycle in females co-varies with males such that and peak levels of testosterone are present during pre-laying and remain high during egg-laying.34-36 Our correlations between male and female aggression scores suggest several new hypotheses. Perhaps aggressive males attract aggressive females through assortative mating; however other traits are likely to be more important for mate choice decisions.37 A more likely explanation is the shared social environment of the breeding pair. In many species, testosterone in females is also strongly correlated with territorial aggression.38-40
Do aggressive responses depend on STI?
A significantly greater aggressive response was directed towards the caged living house sparrow than toward the prepared skin decoy; both adults spent more time attacking the live challenger. In addition, the prepared skin decoy elicited significantly more frequent vocalizations from the Eastern Bluebirds than the live decoy. A living bird likely provides cues beyond the visual and auditory cues provided in this study with the prepared skin decoy and song recordings allowing the adults to accurately identify the intruder. We only detected a correlation between testosterone and aggression in the live caged bird, providing further evidence that that we weren’t accurately measuring aggressive behavior when we used a prepared skin decoy. These concerns have been raised by others who noted that the methods used during STIs, e.g. living versus stuffed decoys, length of stimulation; behavior of living decoy may affect experimental outcomes and confound meaningful interpretation.41
In conclusion, we have shown that a conspecific competitor stimulated aggression more strongly than a heterospecific competitor which is consistent with the assumption that conspecific competition is more important for the evolution of aggressive behavior than heterospecific competition. The influence of heterospecific competition cannot be completely discounted because testosterone and aggression positively correlated for all STIs albeit weakly. Our study raises concern about using stuffed decoys to simulate territorial intrusions because the greatest responses were stimulated by the living heterospecific decoy. We propose that the influence of heterospecific competitors should be studied in more detail using more effective STI methods.
This research was supported by the U.S. Department of Education through the McNair Scholars Program. Melissa Meierhofer provided invaluable assistance in the field.
The author declares no conflict of interest.

©2016 Arboleda, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.